Abstract
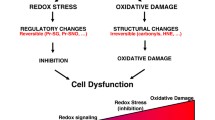
The widely accepted oxidative stress theory of aging postulates that aging results from accumulation of oxidative damage. A prediction of this theory is that, among species, differential rates of aging may be apparent on the basis of intrinsic differences in oxidative damage accrual. Although widely accepted, there is a growing number of exceptions to this theory, most contingently related to genetic model organism investigations. Proteins are one of the prime targets for oxidative damage and cysteine residues are particularly sensitive to reversible and irreversible oxidation. The adaptation and survival of cells and organisms requires the ability to sense proteotoxic insults and to coordinate protective cellular stress response pathways and chaperone networks related to protein quality control and stability. The toxic effects that stem from the misassembly or aggregation of proteins or peptides, in any cell type, are collectively termed proteotoxicity. Despite the abundance and apparent capacity of chaperones and other components of homeostasis to restore folding equilibrium, the cell appears poorly adapted for chronic proteotoxic stress which increases in cancer, metabolic and neurodegenerative diseases. Pharmacological modulation of cellular stress response pathways has emerging implications for the treatment of human diseases, including neurodegenerative disorders, cardiovascular disease, and cancer. A critical key to successful medical intervention is getting the dose right. Achieving this goal can be extremely challenging due to human inter-individual variation as affected by age, gender, diet, exercise, genetic factors and health status. The nature of the dose response in and adjacent to the therapeutic zones, over the past decade has received considerable advances. The hormetic dose–response, challenging long-standing beliefs about the nature of the dose–response in a lowdose zone, has the potential to affect significantly the design of pre-clinical studies and clinical trials as well as strategies for optimal patient dosing in the treatment of numerous diseases. Given the broad cytoprotective properties of the heat shock response there is now strong interest in discovering and developing pharmacological agents capable of inducing stress responses, including carnitines. This paper describes in mechanistic detail how hormetic dose responses are mediated for endogenous cellular defense pathways, including the possible signaling mechanisms by which the carnitine system, by interplaying metabolism, mitochondrial energetics and activation of critical vitagenes, modulates signal transduction cascades that confer cytoprotection against chronic degenerative damage associated to aging and neurodegenerative disorders.






Similar content being viewed by others
References
Balch WE, Morimoto RI, Dillin A, Kelly JW (2008) Adapting proteostasis for disease intervention. Science 319:916–919
Calabrese V, Cornelius C, Dinkova-Kostova AT, Calabrese EJ, Mattson MP (2010) Cellular stress responses, the hormesis paradigm and vitagenes: novel targets for therapeutic intervention in neurodegenerative disorders. Antioxid Redox Signal 610:285–308
Calabrese V, Cornelius C, Dinkova-Kostova AT, Calabrese EJ (2009) Vitagenes, cellular stress response and acetylcarnitine: relevance to hormesis. Biofactors 35:146–160
Gerschman R, Gilbert DL, Nye SW, Dwyer P, Fenn WO (1954) Oxygen poisoning and x-irradiation: a mechanism in common. Science 119:623–626
Gerschman R, Gilbert DL, Nye SW, Fenn WO (1954) Influence of x-irradiation on oxygen poisoning in mice. Proc Soc Exp Biol Med 86:27–29
Harman D (1956) Aging: a theory based on free radical and radiation chemistry. J Gerontol 11:298–300
Harman D (1972) The biologic clock: the mitochondria? J Am Geriatr Soc 20:145–147
Speakman JR (2005) Body size, energy metabolism and lifespan. J Exp Biol 208:1717–1730
Gilbert DL, Colton CA (1999) Reactive oxygen species in biological systems: an interdisciplinary approach. Kluwer, New York
Bokov A, Chaudhuri A, Richardson A (2004) The role of oxidative damage and stress in aging. Mech Ageing Dev 125:811–826
Sohal R, Weindruch R (1996) Oxidative stress, caloric restriction, and aging. Science 273:59–63
Muller FL, Lustgarten MS, Jang Y, Richardson A, Van Remmen H (2007) Trends in oxidative aging theories. Free Radic Biol Med 43:477–503
Salmon AB, Richardson A, Pérez VI (2010) Update on the oxidative stress theory of aging: does oxidative stress play a role in aging or healthy aging? Free Radic Biol Med 48:642–655
Calabrese V, Giuffrida Stella AM, Butterfield DA, Scapagnini G (2004) Redox regulation in neurodegeneration and longevity: role of the heme oxygenase and HSP70 systems in brain stress tolerance. Antioxid Redox Signal 6:895–913
Calabrese V, Butterfield DA, Scapagnini G, Stella AM, Maines MD (2006) Redox regulation of heat shock protein expression by signaling involving nitric oxide and carbon monoxide: relevance to brain aging, neurodegenerative disorders, and longevity. Antioxid Redox Signal 8:444–477
Calabrese V, Guagliano E, Sapienza M, Panebianco M, Calafato S, Puleo E, Pennisi G, Mancuso C, Butterfield AD, Giuffrida Stella AM (2007) Redox regulation of cellular stress response in aging and neurodegenerative disorders: role of vitagenes. Neurochem Res 32:757–773
Sohal RS, Toroser D, Brégère C, Mockett RJ, Orr WC (2008) Age-related decrease in expression of mitochondrial DNA encoded subunits of cytochrome c oxidase in Drosophila melanogaster. Mech Ageing Dev 129:558–561
Tudek B, Winczura A, Janik J, Siomek A, Foksinski M, Oliński R (2010) Involvement of oxidatively damaged DNA and repair in cancer development and aging. Am J Transl Res 2:254–284
Rai P (2010) Oxidation in the nucleotide pool, the DNA Damage response and cellular senescence: defective bricks build a defective house. Mutat Res. [Epub ahead of print]
Mangialasche F, Polidori MC, Monastero R, Ercolani S, Camarda C, Cecchetti R, Mecocci P (2009) Biomarkers of oxidative and nitrosative damage in Alzheimer’s disease and mild cognitive impairment. Ageing Res Rev 8:285–305
Lezza AM, Mecocci P, Cormio A, Beal MF, Cherubini A, Cantatore P, Senin U, Gadaleta MN (1999) Mitochondrial DNA 4977 bp deletion and OH8dG levels correlate in the brain of aged subjects but not Alzheimer’s disease patients. FASEB J 13:1083–1088
Wilson DM 3rd, Bohr VA, McKinnon PJ (2008) DNA damage, DNA repair, ageing and age-related disease. Mech Ageing Dev 129:349–352
Passos JF, Saretzki G, von Zglinicki T (2007) DNA damage in telomeres and mitochondria during cellular senescence: is there a connection? Nucleic Acids Res 35:7505–7513
Niedernhofer LJ, Garinis GA, Raams A, Lalai AS, Robinson AR, Appeldoorn E, Odijk H, Oostendorp R, Ahmad A, van Leeuwen W, Theil AF, Vermeulen W, van der Horst GT, Meinecke P, Kleijer WJ, Vijg J, Jaspers NG, Hoeijmakers JH (2006) A new progeroid syndrome reveals that genotoxic stress suppresses the somatotroph axis. Nature 444:1038–1043
Sohal RS, Orr WC (1992) Relationship between antioxidants, prooxidants, and the aging process. Ann N Y Acad Sci 663:74–84
Stadtman ER (2006) Protein oxidation and aging. Free Radic Res 40:1250–1258
Wang Q, Zhao X, He S, Liu Y, An M, Ji J (2010) Differential proteomics analysis of specific carbonylated proteins in the temporal cortex of aged rats: the deterioration of antioxidant system. Neurochem Res 35:13–21
Chen JJ, Lin F, Qin ZH (2008) The roles of the proteasome pathway in signal transduction and neurodegenerative diseases. Neurosci Bull 24:183–194
Grimm S, Hoehn A, Davies KJ, Grune T (2010) Protein oxidative modifications in the ageing brain: Consequence for the onset of neurodegenerative disease. Free Radic Res. [Epub ahead of print]
Lodi R, Tonon C, Calabrese V, Schapira AH (2006) Friedreich’s ataxia: from disease mechanisms to therapeutic interventions. Antioxid Redox Signal 8:438–443
Calabrese V, Cornelius C, Mancuso C, Pennisi G, Calafato S, Bellia F, Bates TE, Giuffrida Stella AM, Schapira T, Dinkova Kostova AT, Rizzarelli E (2008) Cellular stress response: a novel target for chemoprevention and nutritional neuroprotection in aging, neurodegenerative disorders and longevity. Neurochem Res 33:2444–2471
Calabrese V (2007) Highlight commentary on “redox proteomics analysis of oxidatively 3 modified proteins in G93A–SOD1 transgenic mice—a model of 4 familial amyotrophic lateral sclerosis”. Free Radic Biol Med 43:160–162
Halliwell B (2009) The wanderings of a free radical. Free Radic Biol Med 46:531–542
Seet RC, Lee CY, Lim EC, Tan JJ, Quek AM, Chong WL, Looi WF, Huang SH, Wang H, Chan YH, Halliwell B (2010) Oxidative damage in Parkinson disease: measurement using accurate biomarkers. Free Radic Biol Med 48:560–566
Poon HF, Calabrese V, Calvani M, Butterfield DA (2006) Proteomics analyses of specific protein oxidation and protein expression in aged rat brain and its modulation by l-acetylcarnitine: insights into the mechanisms of action of this proposed therapeutic agent for CNS disorders associated with oxidative stress. Antioxid Redox Signal 8:381–394
Bayot A, Gareil M, Rogowska-Wrzesinska A, Roepstorff P, Friguet B, Bulteau AL (2010) Identification of novel oxidized protein substrates and physiological partners of the mitochondrial ATP-dependent Lon-like protease Pim1. J Biol Chem 285:11445–11457
Ugarte N, Petropoulos I, Friguet B (2010) Oxidized mitochondrial protein degradation and repair in aging and oxidative stress. Antioxid Redox Signal 13:539–549
Sultana R, Perluigi M, Newman SF, Pierce WM, Cini C, Coccia R, Butterfield DA (2010) Redox proteomic analysis of carbonylated brain proteins in mild cognitive impairment and early Alzheimer’s disease. Antioxid Redox Signal 12:327–336
Calabrese V, Mancuso C, Ravagna A, Perluigi M, Cini C, De Marco C, Butterfield DA, Giuffrida Stella AM (2007) In vivo induction of heat shock proteins in the substantia nigra following l-DOPA administration is associated with increased activity of mitochondrial complex I and nitrosative stress in rats: regulation by glutathione redox state. J Neurochem 101:709–717
Bellia F, Calabrese V, Guarino F, Cavallaro M, Cornelius C, De Pinto V, Rizzarelli E (2009) Carnosinase levels in aging brain: redox state induction and cellular stress response. Antioxid Redox Signal 11:2759–2775
Calabrese V, Mancuso C, Calvani M, Rizzarelli E, Butterfield DA, Giuffrida Stella AM (2007) Nitric oxide in the CNS: neuroprotection versus neurotoxicity. Nature Neurosi 8:766–775
Calabrese V, Signorile A, Cornelius C, Mancuso C, Scapagnini G, Ventimiglia B, Ragusa N, Dinkova-Kostova A (2008) Practical approaches to investigate redox regulation of heat shock protein expression and intracellular glutathione redox state. Methods Enzymol 441:83–110
Calabrese V, Colombrita C, Sultana R, Scapagnini G, Calvani M, Butterfield DA, Stella AM (2006) Redox modulation of heat shock protein expression by acetylcarnitine in aging brain: relationship to antioxidant status and mitochondrial function. Antioxid Redox Signal 8:404–416
Perluigi M, Di Domenico F, Butterfield DA, Giorgi A, Schininà ME, Coccia R, Cini C, Bellia F, Cambria MT, Cormelius C, Calabrese V (2010) Redox proteomics in aging rat brain: involvement of mitochondrial GSH status and mitochondrial protein oxidation in the aging process. J Neurosci Res (in press)
Bradley MA, Markesbery WR, Lovell MA (2010) Increased levels of 4-hydroxynonenal and acrolein in the brain in preclinical Alzheimer disease. Free Radic Biol Med 48:1570–1576
Sultana R, Butterfield DA (2010) Proteomics identification of carbonylated and HNE-bound brain proteins in Alzheimer’s disease. Methods Mol Biol 566:123–135
Lovell MA, Xie C, Markesbery WR (1998) Decreased glutathione transferase activity in brain and ventricular fluid in Alzheimer’s disease. Neurology 51:1562–1566
Van Raamsdonk JM, Meng Y, Camp D, Yang W, Jia X, Bénard C, Hekimi S (2010) Decreased energy metabolism extends life span in Caenorhabditis elegans without reducing oxidative damage. Genetics 185:559–571
Van Raamsdonk JM, Hekimi S (2010) Reactive oxygen species and aging in caenorhabditis elegans: causal or casual relationship? Antioxid Redox Signal
Calabrese V, Scapagnini G, Giuffrida Stella AM, Bates TE, Clark JB (2001) Mitocondrial involvement in brain function and dysfunction: relevance to aging, neurodegenerative disordes and longevity. Neurochem Res 26:739–764
Druzhyna NM, Wilson GL, LeDoux SP (2008) Mitochondrial DNA repair in aging and disease. Mech Age Dev 129:383–390
Hamm-Alvarez S, Cadenas E (2009) Mitochondrial medicine and mitochondrion-based therapeutics. Adv Drug Deliv Rev 60:1437–1438
Hamm-Alvarez S, Cadenas E (2009) Mitochondrial medicine and therapeutics, Part II. Adv Drug Deliv Rev 61:1233
Yap LP, Garcia JV, Han D, Cadenas E (2009) The energy-redox axis in aging and age-related neurodegeneration. Adv Drug Deliv Rev 61:1283–1298
Schapira AH (2002) Primary and secondary defects of the mitochondrial respiratory chain. J Inherit Metab Dis 25:207–214
Tonon C, Lodi R (2008) Idebenone in Friedreich’s ataxia. Expert Opin Pharmacother 9:2327–2337
Calabrese V, Scapagnini G, Colombrita C, Ravagna A, Pennisi G, Giuffrida Stella AM, Galli F, Butterfield DA (2003) Redox regulation of heat shock protein expression in aging and neurodegenerative disorders associated with oxidative stress: a nutritional approach. Amino Acids 25:437–444
Petrosillo G, Matera M, Casanova G, Ruggiero FM, Paradies G (2008) Mitochondrial dysfunction in rat brain with aging Involvement of complex I, reactive oxygen species and cardiolipin. Neurochem Int 53:126–131
Ames BN, Liu J (2004) Delaying the mitochondrial decay of aging with acetylcarnitine. Ann N Y Acad Sci 1033:108–116
Hempenstall S, Picchio L, Mitchell SE, Speakman JR, Selman C (2010) The impact of acute caloric restriction on the metabolic phenotype in male C57BL/6 and DBA/2 mice. Mech Ageing Dev 131:111–118
Mattson MP (2008) Dietary factors, hormesis and health. Ageing Res Rev 7:43–48
Ristow M, Zarse K (2010) How increased oxidative stress promotes longevity and metabolic health: the concept of mitochondrial hormesis (mitohormesis). Exp Gerontol 45:410–418
Benard G, Faustin B, Passerieux E, Galinier A, Rocher C, Bellance N, Delage JP, Casteilla L, Letellier T, Rossignol R (2006) Physiological diversity of mitochondrial oxidative phosphorylation. Am J Physiol Cell Physiol 291:1172–1182
Luft R, Ikkos D, Palmieri G, Ernster L, Afzelius A (1962) A case of severe hypermetabolism of nonthyroid origin with a defect in the maintenance of mitochondrial respiratory control: a correlated clinical, biochemical and morphological study. J Clin Invest 41:1776–1804
Schapira AH (2008) Mitochondrial dysfunction in neurodegenerative diseases. Neurochem Res 33:2502–2509
Wallace DC (1999) Mitochondrial diseases in man and mouse. Science 283:1482–1488
Wallace DC (2008) Mitochondria as Chi. Genetics 179:727–735
Wang Y, Michikawa Y, Mallidis C, Bai Y, Woodhouse L, Yarasheski KE, Miller CA, Askanas V, Engel WK, Bhasin S, Attardi G (2001) Muscle-specific mutations accumulate with aging in critical human mtDNA control sites for replication. Proc Natl Acad Sci U S A 98:4022–4027
Mazat J, Rossignol R, Malgat M, Rocher C, Faustin B, Letellier T (2001) What do mitochondrial diseases teach us about normal mitochondrial functions that we already knew: threshold expression of mitochondrial defects. Biochim Biophys Acta 1504:20–30
Letellier T, Malgat M, Rossignol R, Mazat JP (1998) Metabolic control analysis and mitochondrial pathologies. Mol Cell Biochem 184:409–417
Davey GP, Peuchen S, Clark JB (1998) Energy thresholds in brain mitochondria. J Biol Chem 273:12753–12757
Pathak RU, Davey GP (2008) Complex I and energy thresholds in the brain. Biochim Biophys Acta 1777:777–782
Benard G, Bellance N, James D, Parrone P, Fernandez H, Letellier T, Rossignol R (2007) Mitochondrial bioenergetics and structural network organization. J Cell Sci 120:838–848
Rocher C, Taanman JW, Pierron D, Faustin B, Benard G, Rossignol R, Malgat M, Pedespan L, Letellier T (2008) Influence of mitochondrial DNA level on cellular energy metabolism: implications for mitochondrial diseases. J Bioenerg Biomembr 40:59–67
Benard G, Faustin B, Galinier A, Rocher C, Bellance N, Smolkova K, Casteilla L, Rossignol R, Letellier T (2008) Functional dynamic compartmentalization of respiratory chain intermediate substrates: implications for the control of energy production and mitochondrial diseases. Int J Biochem Cell Biol 40:1543–1554
Kordower JH, Chu Y, Hauser RA, Freeman TB, Olanow CW (2008) Lewy body-like pathology in long-term embryonic nigral transplants in Parkinson’s disease. Nat Med 14:504–506
Kilbride SM, Telford JE, Tipton KF, Davey GP (2008) Partial inhibition of complex I activity increases Ca(2+)-independent glutamate release rates from depolarized synaptosomes. J Neurochem 106:826–834
Kilbride SM, Telford JE, Davey GP (2008) Age-related changes in H2O2 production and bioenergetics in rat brain synaptosomes. Biochim Biophys Acta 1777:783–788
Keeney PM, Xie J, Capaldi RA, Bennett JP Jr (2006) Parkinson’s disease brain mitochondrial complex I has oxidatively damaged subunits and is functionally impaired and misassembled. J Neurosci 26:5256–5264
Vogel RO, Smeitink JA, Nijtmans LG (2007) Human mitochondrial complex I assembly: a dynamic and versatile process. Biochim Biophys Acta 1767:1215–1227
Vogel RO, van den Brand MA, Rodenburg RJ, van den Heuvel LP, Tsuneoka M, Smeitink JA, Nijtmans LG (2007) Investigation of the complex I assembly chaperones B17.2L and NDUFAF1 in a cohort of CI deficient patients. Mol Genet Metab 91:176–182
Dudkina NV, Kouřil R, Peters K, Braun HP, Boekema EJ (2010) Structure and function of mitochondrial supercomplexes. Biochim Biophys Acta 1797:664–670
Wittig I, Schägger H (2009) Supramolecular organization of ATP synthase and respiratory chain in mitochondrial membranes. Biochim Biophys Acta 1787:672–680
Forquer I, Covian R, Bowman MK, Trumpower BL, Kramer DM (2006) Similar transition states mediate the Q-cycle and superoxide production by the cytochrome bc1 complex. J Biol Chem 281:38459–38465
Bilban M, Haschemi A, Wegiel B, Chin BY, Wagner O, Otterbein LE (2008) Heme oxygenase and carbon monoxide initiate homeostatic signaling. J Mol Med 86:267–279
Calabrese V, Cornelius C, Rizzarelli E, Owen JB, Dinkova-Kostova AT, Butterfield DA (2009) Nitric oxide in cell survival: a Janus molecule. Antioxid Redox Signal 11:2717–2739
Wang W, Fang H, Groom L, Cheng A, Zhang W, Liu J, Wang X, Li K, Han P, Zheng M, Yin J, Wang W, Mattson MP, Kao JP, Lakatta EG, Sheu SS, Ouyang K, Chen J, Dirksen RT, Cheng H (2008) Superoxide flashes in single mitochondria. Cell 134:279–290
McCord JM (2002) Superoxide dismutase in aging and disease: an overview. Methods Enzymol 349:331–341
Cho DH, Nakamura T, Lipton SA (2010) Mitochondrial dynamics in cell death and neurodegeneration. Cell Mol Life Sci. 2010 Jun 25. [Epub ahead of print]
Nakamura T, Cieplak P, Cho DH, Godzik A, Lipton SA (2010) S-Nitrosylation of Drp1 links excessive mitochondrial fission to neuronal injury in neurodegeneration. Mitochondrion 10:573–578
Gu Z, Nakamura T, Lipton SA (2010) Redox reactions induced by nitrosative stress mediate protein misfolding and mitochondrial dysfunction in neurodegenerative diseases. Mol Neurobiol 41:55–72
Nakamura T, Lipton SA (2010) Redox regulation of mitochondrial fission, protein misfolding, synaptic damage, and neuronal cell death: potential implications for Alzheimer’s and Parkinson’s diseases. Apoptosis [Epub ahead of print]
Büeler H (2010) Mitochondrial dynamics, cell death and the pathogenesis of Parkinson’s disease. Apoptosis [Epub ahead of print]
Knott AB, Perkins G, Schwarzenbacher R, Bossy-Wetzel E (2008) Mitochondrial fragmentation in neurodegeneration. Nat Rev Neurosci 9:505–518
Bonda DJ, Wang X, Perry G, Smith MA, Zhu X (2010) Mitochondrial dynamics in Alzheimer’s disease: opportunities for future treatment strategies. Drugs Aging 27:181–192
Gottlieb RA, Carreira RS (2010) Autophagy in health and disease. 5. Mitophagy as a way of life. Am J Physiol Cell Physiol 299:C203–210
Rosca MG, Lemieux H, Hoppel CL (2009) Mitochondria in the elderly: is acetylcarnitine a rejuvenator? Adv Drug Deliv Rev 61:1332–1342
Piantadosi CA (2008) Carbon monoxide, reactive oxygen signaling, and oxidative stress. Free Radic Biol Med 45:562–569
Piantadosi CA, Carraway MS, Babiker A, Suliman HB (2008) Heme oxygenase-1 regulates cardiac mitochondrial biogenesis via Nrf2-mediated transcriptional control of nuclear respiratory factor-1. Circ Res 103:1232–1240
Schulz H (1888) Uber Hefegifte. Pfluger’s Arch Ges Physiol 42:517–541
Calabrese EJ (1999) Evidence that hormesis represents an “overcompensation” response to a disruption in homeostasis. Ecotoxicol Environ Safety 42:135–137
Mitchel REJ (2007) Low doses of radiation reduce risk in vivo. Dose Response 5:1–10
Lacassagne A, Buu-Hoi NP, Rudali G (1945) Induction of the carcinogenic action produced by a weakly carcinogenic hydrocarbon on a highly active carcinogenic hydrocarbon. Br J Exp Pathol 26:5–12
Sykes PJ, Morley AA, Hooker AM (2006) The pKZ1 recombination mutation assay: a sensitive assay for low dose studies. Dose Response 4:91–105
Boreham DR, Dolling J-A, Somers C, Quinn J, Mitchel REJ (2006) The adaptive response and protection against heritable mutations and fetal malformation. Dose Response 4:317–326
Mothersill C, Seymour CB (2006) Radiation-induced bystander effects and the DNA paradigm: an “out of field” perspective. Mutat Res 597:5–10
Sakai K, Nomura T, Ina Y (2006) Enhancement of bio-protective functions by low dose/dose-rate radiation. Dose Response 4:327–332
Scott BR, DiPalma J (2006) Sparsely ionizing diagnostic and natural background radiations are likely preventing cancer and other genomic-instability-associated diseases. Dose Response 5:230–255
Redpath JL (2006) Suppression of neoplastic transformation in vitro by low doses of low LET radiation. Dose Response 4:302–308
Calabrese EJ, Baldwin LA (2000) Chemical hormesis: Its historical foundations as a biological hypothesis. Hum Exp Toxicol 19:2–31
Calabrese EJ, Baldwin LA (2000) The marginalization of hormesis. Hum Exp Toxicol 19:32–40
Calabrese EJ, Baldwin LA (2000) Radiation hormesis: its historical foundations as a biological hypothesis. Hum Exp Toxicol 19:41–75
Calabrese EJ, Baldwin LA (2000) Radiation hormesis: the demise of a legitimate hypothesis. Hum Exp Toxicol 19:76–84
Calabrese EJ, Baldwin LA (2003) Ethanol and hormesis. Crit Rev Toxicol 33:407–424
Calabrese EJ (2008) Hormesis. In: Melnick E, Everitt B (eds) Encyclopedia of quantitative risk assessment and analysis. Wiley, Chichester, pp 838–844
Calabrese EJ (2009) The road to linearity: shy linearity at low doses became the basis for carcinogen risk assessment. Arch Toxicol 83:203–225
Stebbing ARD (1976) Effects of low metal levels on a clonal hydroid. J Mar Biol Assoc UK 56:977–994
Szabadi E (1977) Model of 2 functionally antagonistic receptor populations activated by same agonist. J Theor Biol 69:101–112
Luckey TD (1992) Radiation hormesis. CRC, Boca Raton
Calabrese EJ, Baldwin LA (2000) Tales of two similar hypotheses: the rise and fall of chemical and radiation hormesis. Hum Exp Toxicol 19:85–97
Calabrese EJ, Blain R (2005) The occurrence of hormetic dose responses in the toxicological literature, the hormesis database: an overview. Toxicol Appl Pharmacol 202:289–301
Mitchel REJ (2006) Low doses of radiation are protective in vitro and in vivo: evolutionary origins. Dose Response 4:75–90
Thong H-Y, Maibach HI (2008) Hormesis [biological effects of low level exposure (BELLE)] and dermatology. Dose Response 6:1–15
Eaton DL, Klaassen CD (2003) Principles of toxicology. In: Casarett & Doull’s essentials of toxicology, Chap 2. The McGraw-Hill Companies, Inc. pp. 6–20
Calabrese EJ, Ricci PF (2010) Hormesis in environmental health: how hormesis will change the risk assessment process. Encycl Environ Health (in press)
Calabrese EJ (1999) Evidence that hormesis represents an “overcompensation” response to a disruption in homeostasis. Ecotoxicol Environ Saf 42:135–137
Calabrese EJ, Blain RB (2009) Hormesis and plant biology. Environ Poll. 157:42–48
Calabrese EJ, Baldwin LA (2003) Inorganics and hormesis. Crit Rev Toxicol 33:215–304
Calabrese EJ, Baldwin LA (2003) Chemotherapeutics and hormesis. Crit Rev Toxicol 33:305–354
Calabrese EJ, Baldwin LA (2003) Peptides and hormesis. Crit Rev Toxicol 33:355–406
Calabrese EJ, Bachmann KA, Bailer AJ, Bolger PM, Borak J, Cai L, Cedergreen N, Cherian MG, Chiueh CC, Clarkson TW, Cook RR, Diamond DM, Doolittle DJ, Dorato MA, Duke SO, Feinendegen L, Gardner DE, Hart RW, Hastings KL, Hayes AW, Hoffmann GR, Ives JA, Jaworowski Z, Johnson TE, Jonas WB, Kaminski NE, Keller JG, Klaunig JE, Knudsen TB, Kozumbo WJ, Lettieri T, Liu SZ, Maisseu A, Maynard KI, Masoro EJ, McClellan RO, Mehendale HM, Mothersill C, Newlin DB, Nigg HN, Oehme FW, Phalen RF, Philbert MA, Rattan SI, Riviere JE, Rodricks J, Sapolsky RM, Scott BR, Seymour C, Sinclair DA, Smith-Sonneborn J, Snow ET, Spear L, Stevenson DE, Thomas Y, Tubiana M, Williams GM, Mattson MP (2007) Biological stress response terminology: Integrating the concepts of adaptive response and preconditioning stress within a hormetic dose-response framework. Toxicol Appl Pharmacol 222:122–128
Flood JF, Smith GE, Cherkin A (1982) Memory retention—enhancement by cholinergic drug-combinations in mice. Gerontologist 22:230–231
Flood JF, Smith GE, Cherkin A (1983) Memory retention—potentiation of cholinergic drug-combinations in mice. Neurobiol Aging 4:37–43
Flood JF, Smith GE, Cherkin A (1984) Memory retention—enhancement by synergistic oral cholinergic drug-combination in mice. Gerontologist 24:149–158
Flood JF, Smith GE, Cherkin A (1985) Memory enhancement—supra-additive effect of subcutaneous chlolinergic drug-combinations in mice. Psychopharmacology 86:61–67
Calabrese EJ (2008) Neuroscience and hormesis: overview and general findings. Crit Rev Toxicol 38:249–252
Calabrese EJ, Baldwin LA (2002) Hormesis and high risk groups. Regul Toxicol Pharmacol 35:14–428
Calabrese EJ (2005) Cancer biology and hormesis: human tumor cell lines commonly display hormetic (biphasic) dose responses. Crit Rev Toxicol 35:463–582
Randall WA, Price CW, Welch H (1947) Demonstration of hormesis (increase in fatality rate) by penicillin. Am J Pub Health 37:421–425
Welch H, Price CW, Randall WA (1946) Increase in fatality rate of E. Typhosa for white mice by streptomycin. J Am Pharm 35:155–158
Abramowitz J, Dai C, Hirschi KK, Dmitieva RI, Doris PA, Liu L, Allen JC (2003) Ouabain- and marinobufagenin-induced proliferation of human umbilical vein smooth muscle cells and a rat vascular smooth muscle cell lines, A7r5. Circulation 108:3048–3053
Chueh S-C, Guh J-H, Chen J, Lai M-K, Teng C-M (2001) Dual effects of ouabain on the regulation of proliferation and apoptosis in human prostatic smooth muscle cells. J Urol 166:347–353
Gerich FJ, Funke F, Hildebrandt B, Faßhauer M, Müller M (2009) H2O2-mediated modulation of cytosolic signaling and organelle function in rat hippocampus. Pflugers Arch 458:937–952
Huddleston AT, Tang W, Takeshima H, Hamilton SL, Klann E (2008) Superoxide-induced potentiation in the hippocampus requires activation of ryanodine receptor type 3 and ERK. J Neurophysiol 99:1565–1571
Gopalakrishna R, Gundimeda U, Schiffman JE, McNeill TH (2008) A direct redox regulation of protein kinase C isoenzymes mediates oxidant-induced neuritogenesis in PC12 cells. J Biol Chem 283:14430–14444
Terada LS (2006) Specificity in reactive oxidant signaling: think globally, act locally. J Cell Biol 174:615–623
Mattson MP, Goodman Y, Luo H, Fu W, Furukawa K (1997) Activation of NF-kappaB protects hippocampal neurons against oxidative stress-induced apoptosis: evidence for induction of manganese superoxide dismutase and suppression of peroxynitrite production and protein tyrosine nitration. J Neurosci Res 49:681–697
Bruce-Keller AJ, Geddes JW, Knapp PE, McFall RW, Keller JN, Holtsberg FW, Parthasarathy S, Steiner SM, Mattson MP (1999) Anti-death properties of TNF against metabolic poisoning: mitochondrial stabilization by MnSOD. J Neuroimmunol 93:53–71
Ekdahl CT, Kokaia Z, Lindvall O (2009) Brain inflammation and adult neurogenesis: the dual role of microglia. Neuroscience 158:1021–1029
Jiang F, Zhang ZG, Katakowski M, Robin AM, Faber M, Zhang F, Chopp M (2004) Angiogenesis induced by photodynamic therapy in normal rat brains. Photochem Photobiol 79:494–498
Bandopadhyay R, de Belleroche J (2010) Pathogenesis of Parkinson’s disease: emerging role of molecular chaperones. Trends Mol Med 16:27–36
Morimoto RI (2008) Proteotoxic stress and inducible chaperone networks in neurodegenerative disease and aging. Genes Dev 22:1427–1438
Csermely P, Söti C, Blatch GL (2007) Chaperones as parts of cellular networks. Adv Exp Med Biol 594:55–63
Amijee H, Madine J, Middleton DA, Doig AJ (2009) Inhibitors of protein aggregation and toxicity. Biochem Soc Trans 37:692–696
Mattson MP, Magnus T (2006) Ageing and neuronal vulnerability. Nat Rev Neurosci 7:278–294
Saibil HR (2008) Chaperone machines in action. Curr Opin Struct Biol 18:35–42
Glabe CG (2008) Structural classification of toxic amyloid oligomers. J Biol Chem 283:29639–29643
Broadley SA, Hartl FU (2009) The role of molecular chaperones in human misfolding diseases. FEBS Lett 583:2647–2653
Gidalevitz T, Kikis EA, Morimoto RI (2010) A cellular perspective on conformational disease: the role of genetic background and proteostasis networks. Curr Opin Struct Biol 20:23–32
Nassif M, Matus S, Castillo K, Hetz C (2010) Amyotrophic lateral sclerosis pathogenesis: a journey through the secretory pathway. Antioxid Redox Signal. [Epub ahead of print]
Eizirik DL, Cardozo AK, Cnop M (2008) The role for endoplasmic reticulum stress in diabetes mellitus. Endocr Rev 29:42–61
Hoozemans JJ, van Haastert ES, Nijholt DA, Rozemuller AJ, Eikelenboom P, Scheper W (2009) The unfolded protein response is activated in pretangle neurons in Alzheimer’s disease hippocampus. Am J Pathol 174:1241–1251
Scheper W, Hoozemans JJ (2009) Endoplasmic reticulum protein quality control in neurodegenerative disease: the good, the bad and the therapy. Curr Med Chem 16:615–626
Naidoo N (2009) ER and aging-protein folding and the ER stress response. Ageing Res Rev 8:150–159
Schröder M (2008) Endoplasmic reticulum stress responses. Cell Mol Life Sci 65:862–894
Verkhratsky A (2004) Endoplasmic reticulum calcium signaling in nerve cells. Biol Res 37:693–699
Jiao J, Huang X, Feit-Leithman RA, Neve RL, Snider W, Dartt DA, Chen DF (2005) Bcl-2 enhances Ca(2+) signaling to support the intrinsic regenerative capacity of CNS axons. EMBO J 24:1068–1078
Kitao Y, Ozawa K, Miyazaki M, Tamatani M, Kobayashi T, Yanagi H, Okabe M, Ikawa M, Yamashima T, Stern DM, Hori O, Ogawa S (2001) Expression of the endoplasmic reticulum molecular chaperone (ORP150) rescues hippocampal neurons from glutamate toxicity. J Clin Invest 108:1439–1450
Um HS, Kang EB, Leem YH, Cho IH, Yang CH, Chae KR, Hwang DY, Cho JY (2008) Exercise training acts as a therapeutic strategy for reduction of the pathogenic phenotypes for Alzheimer’s disease in an NSE=APPsw-transgenic model. Int J Mol Med 22:529–539
Matus S, Lisbona F, Torres M, León C, Thielen P, Hetz C (2008) The stress rheostat: an interplay between the unfolded protein response (UPR) and autophagy in neurodegeneration. Curr Mol Med 8:157–172
Sado M, Yamasaki Y, Iwanaga T, Onaka Y, Ibuki T, Nishihara S, Mizuguchi H, Momota H, Kishibuchi R, Hashimoto T, Wada D, Kitagawa H, Watanabe TK (2009) Protective effect against Parkinson’s disease-related insults through the activation of XBP1. Brain Res 1257:16–24
Madeo F, Eisenberg T, Kroemer G (2009) Autophagy for the avoidance of neurodegeneration. Genes Dev 23:2253–2259
Pennuto M, Tinelli E, Malaguti M, Del Carro U, D’Antonio M, Ron D, Quattrini A, Feltri ML, Wrabetz L (2008) Ablation of the UPR-mediator CHOP restores motor function and reduces demyelination in Charcot-Marie-Tooth 1B mice. Neuron 57:393–405
Sakaki K, Wu J, Kaufman RJ (2008) Protein kinase Cu is required for autophagy in response to stress in the endoplasmic reticulum. J Biol Chem 283:15370–15380
Schulz TJ, Zarse K, Voigt A, Urban N, Birringer M, Ristow M (2007) Glucose restriction extends Caenorhabditis elegans life span by inducing mitochondrial respiration and increasing oxidative stress. Cell Metab 6:280–293
Azad MB, Chen Y, Gibson SB (2009) Regulation of autophagy by reactive oxygen species (ROS): implications for cancer progression and treatment. Antioxid Redox Signal 11:777–790
Fujioka Y, Noda NN, Nakatogawa H, Ohsumi Y, Inagaki F (2010) Dimeric coiled-coil structure of Saccharomyces cerevisiae Atg16 and its functional significance in autophagy. J Biol Chem 285:1508–1515
Jakubowicz-Gil J, Langner E, Wertel I, Piersiak T, Rzeski W (2010) Temozolomide, quercetin and cell death in the MOGGCCM astrocytoma cell line. Chem Biol Interact [Epub ahead of print]
Yogev O, Goldberg R, Anzi S, Yogev O, Shaulian E (2010) Jun proteins are starvation-regulated inhibitors of autophagy. Cancer Res 70:2318–2327
Luk KC, Mills IP, Trojanowski JQ, Lee VM (2008) Interactions between Hsp70 and the hydrophobic core of alpha-synuclein inhibit fibril assembly. Biochemistry 47:12614–12625
Rump TJ, Muneer PM, Szlachetka AM, Lamb A, Haorei C, Alikunju S, Xiong H, Keblesh J, Liu J, Zimmerman MC, Jones J, Donohue TM Jr, Persidsky Y, Haorah J (2010) Acetyl-l-carnitine protects neuronal function from alcohol-induced oxidative damage in the brain. Free Radic Biol Med 49:1494–1504
Calabrese V, Stella AMG, Calvani M, Butterfield DA (2006) Acetylcarnitine and cellular stress response: roles in nutritional redox homeostasis andregulation of longevity genes. J Nutr Biochem 17:73–88
Jones LL, McDonald D, Borum PR (2009) Acylcarnitines: role in brain. Progr Lipid Res
Calabrese V, Rizza V (1999) Formation of propionate after short-term ethanol treatment and its interaction with the carnitine pool in rat. Alcohol 19:169–176
Calabrese V, Calvani M, Butterfield DA (2004) Increased formation of short-chain organic acids after chronic ethanol administration and its interaction with the carnitine pool in rat. Arch Biochem Biophys 431:271–278
Noland RC, Koves TR, Seiler SE, Lum H, Lust RM, Ilkayeva O, Stevens RD, Hegardt FG, Muoio DM (2009) Carnitine insufficiency caused by aging and overnutrition compromises mitochondrial performance and metabolic control. J Biol Chem 284:22840–22852
Bieber LL (1998) Carnitine. Annu Rev Biochem 57:261–283
Evans AM, Fornasini G (2003) Pharmacokinetics of l-carnitine. Clin Pharmacokinet 42:941–967
Rebouche CJ (2004) Kinetics, pharmacokinetics, and regulation of l-carnitine and acetyl-l-carnitine metabolism. Ann N Y Acad Sci 1033:30–41
Steiber A, Kerner J, Hoppel CL (2004) Carnitine: a nutritional, biosynthetic, and functional perspective. Mol Aspects Med 25:455–473
Koves TR, Ussher JR, Noland RC, Slentz D, Mosedale M, Ilkayeva O, Bain J, Stevens R, Dyck JR, Newgard CB, Lopaschuk GD, Muoio DM (2008) Mitochondrial overload and incomplete fatty acid oxidation contribute to skeletal muscle insulin resistance. Cell Metab 7:45–56
Lombard KA, Olson AL, Nelson SE, Rebouche CJ (1989) Carnitine status of lactoovovegetarians and strict vegetarian adults and children. Am J Clin Nutr 50:301–306
Brass EP, Hoppel CL, Hiatt WR (1994) Effect of intravenous l-carnitine on carnitine homeostasis and fuel metabolism during exercise in humans. Clin Pharmacol Ther 55:681–692
Murakami R, Tanaka A, Nakamura H (1997) The effect of starvation on brain carnitine concentration in neonatal rats. J Pediatr Gastroenterol Nutr 25:385–387
Bremer J (1983) Carnitine-metabolism and functions. Physiol Rev 63:1420–1480
Zhao S, Xu W, Jiang W, Yu W, Lin Y, Zhang T, Yao J, Zhou L, Zeng Y, Li H, Li Y, Shi J, An W, Hancock SM, He F, Qin L, Chin J, Yang P, Chen X, Lei Q, Xiong Y, Guan KL (2010) Regulation of cellular metabolism by protein lysine acetylation. Science 327:1000–1004
Alonso-Montes C, Castro MG, Reguero JR, Perrot A, Ozcelik C, Geier C, Posch MG, Morris C, Alvarez V, Ruiz-Ortega M, Coto E (2008) Mitochondrial transcription factor TFA, TFB1 and TFB2: a search for DNA variant/haplotypes and the risk of cardiac hyperthrophy. Dis Markers 25:131–139
Takahashi H, McCaffery JM, Irizarry RA, Boeke JD (2006) Nucleocytosolic acetylcoenzyme a synthetase is required for histone acetylation and global transcription. Mol Cell 23:207–217
Distler AM, Kerner J, Hoppel CL (2007) Post-translational modifications of rat liver mitochondrial outer membrane proteins identified by mass spectrometry. Biochim Biophys Acta 1774:628–636
Infante JP, Huszagh VA (2000) Secondary carnitine deficiency and impaired docosahexaenoic [22:6n–3] acid synthesis: a common denominator in thepathophysiology of diseases of oxidative phosphorylation and beta-oxidation. FEBS Lett 468:1–5
Calabrese V, Ravagna A, Colombrita C, Scapagnini G, Guagliano E, Calvani M, Butterfield DA, Giuffrida Stella AM (2005) Acetylcarnitine induces heme oxygenase in rat astrocytes and protects against oxidative stress: involvement of the transcription factor Nrf2. J Neurosci Res 79:509–521
McDaniel MA, Maier SF, Einstein GO (2003) “Brain-specific” nutrients: a memory cure? Nutrition 19:957–975
Traina G, Federighi G, Brunelli M, Scuri R (2009) Cytoprotective effect of cetyl-l-carnitine evidenced by analysis of gene expression in the rat brain. Mol Neurobiol 39:101–106
Calabrese V, Scapagnini G, Ravagna A, Colombrita C, Spadaro F, Butterfield DA, Giuffrida Stella AM (2004) Increased expression of heat shock proteins in rat brain during aging: relationship with mitochondrial function and glutathione redox state. Mech Ageing Dev 125:325–335
Traina G, Valleggi S, Bernardi R (2004) Identification of differentially expressed genes induced in the rat brain by acetyl-l-carnitine as evidenced by suppression subtractive hybridisation. Mol Brain Res 132:57–63
Traina G, Bernardi R, Rizzo M, Calvani M, Durante M, Brunelli M (2006) Acetyl-l-carnitine up-regulates expression of voltage-dependent anion channel in the rat brain. Neurochem Int 48:673–678
Binienda Z, Virmani A, Przybyla-Zawislak B, Schmued L (2004) Neuroprotective effect of l-carnitine in the 3-nitropropionic acid [3-NPA]-evoked neurotoxicity in rats. Neurosci Lett 367:264–267
Lesnefsky EJ, He D, Moghaddas S, Hoppel CL (2006) Reversal of mitochondrial defects before ischemia protects the aged heart. FASEB J 20:1543–1545
Chiechio S, Copani A, Nicoletti F, Gereau RW 4th (2006) l-acetylcarnitine: a proposed therapeutic agent for painful peripheral neuropathies. Curr Neuropharmacol 4:233–237
Chiechio S, Copani A, Zammataro M, Battaglia G, Gereau RW 4th, Nicoletti F (2010) Transcriptional regulation of type-2 metabotropic glutamate receptors: an epigenetic path to novel treatments for chronic pain. Trends Pharmacol Sci 31:153–160
Di Cesare ML, Ghelardini C, Calvani M, Nicolai R, Mosconi L, Vivoli E, Pacini A, Bartolini A (2007) Protective effect of acetyl-l-carnitine on the apoptotic pathway of peripheral neuropathy. Eur J Neurosci 26:820–827
Watt MJ, Hevener AL (2008) Fluxing the mitochondria to insulin resistance. Cell Metab 7:5–6
Adams SH, Hoppel CL, Lok KH, Zhao L, Wong SW, Minkler PE, Hwang DH, Newman JW, Garvey WT (2009) Plasma acylcarnitine profiles suggest incomplete long-chain fatty acid beta-oxidation and altered tricarboxylic acid cycle activity in type 2 diabetic African-American women. J Nutr 139:1073–1081
Stephens FB, Constantin-Teodosiu D, Greenhaff PL (2007) New insights concerning the role of carnitine in the regulation of fuel metabolism in skeletal muscle. J Physiol 581:431–444
Heo YR, Kang CW, Cha S (2001) l-carnitine changes the levels of insulin-like growth factors (IGFs) and IGF binding proteins in streptozotocin-induced diabetic rat. J Nutr Sci Vitaminol (Tokyo) 47:329–334
Randle PJ, Garland PB, Hales CN, Newsholme EA (1963) The glucose fatty-acid cycle. Its role in insulin sensitivity and the metabolic disturbances of diabetes mellitus. Lancet 1:785–789
Abdul HM, Calabrese V, Calvani M, Butterfield DA (2006) Acetyl-l-carnitine-induced up-regulation of heat shock proteins protects cortical neurons against amyloid-beta peptide 1–42-mediated oxidative stress and neurotoxicity: implications for Alzheimer’s disease. J Neurosci Res 84:398–408
Wallace DC (2010) The epigenome and the mitochondrion: bioenergetics and the environment. Genes Dev 24:1571–1573
Kuhn TS (1996) The structure of scientific revolutions. University of Chicago Press, Chicago
Wallace DC (2007) Why do we still have a maternally inherited mitochondrial DNA? Insights from evolutionary medicine. Annu Rev Biochem 76:781–821
Wallace DC (2010) Mitochondrial DNA mutations in disease and aging. Environ Mol Mutagen 51:440–450
Acknowledgments
Work from the authors’ laboratories was supported by grants from MIUR, FIRB RBRN07BMCT, I.N.B.B., and by ‘‘Fondi Ateneo’’ 2008 and 2009. We are very much honoured to contribute to this Special Issue in honour of Abel Lajtha. Everybody in the field of Neurochemistry knows Abel Lajtha and his great contribution to the advancement of research in Neurochemistry. He has been the Editor in Chief of the Journal “Neurochemical Research” for so many years holding very stimulating and interesting Meetings of the Editorial Board to which we are very proud to have participated. It is difficult to imagine future Editorial Board Meetings without him. We would like to thank him very much for his great contribution to the advancement and success of Neurochemical Research and ask him to continue helping us with its precious efforts and advices.
Author information
Authors and Affiliations
Corresponding author
Additional information
Special issue article in honor of Dr. Abel Lajtha
Rights and permissions
About this article
Cite this article
Calabrese, V., Cornelius, C., Stella, A.M.G. et al. Cellular Stress Responses, Mitostress and Carnitine Insufficiencies as Critical Determinants in Aging and Neurodegenerative Disorders: Role of Hormesis and Vitagenes. Neurochem Res 35, 1880–1915 (2010). https://doi.org/10.1007/s11064-010-0307-z
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11064-010-0307-z