Abstract
Introduction
Glioblastoma (GBM) is the most common and aggressive human primary brain malignancy. The key properties of GBM, stemness and invasiveness, are known to be associated with a highly unfavorable prognosis. Notably, the process of epithelial-mesenchymal transition (EMT) is closely related to the progression of GBM. On the basis of reports that 2′-hydroxycinnamaldehyde (HCA) and its derivative, 2′-benzoyloxycinnamaldehyde (BCA), suppresses EMT in several human cancer cells, we sought to evaluate the therapeutic efficacy of HCA and BCA, alone and in combination with temozolomide (TMZ), on GBM tumorspheres (TSs).
Methods
Two human GBM TSs were treated with HCA, BCA, or TMZ. Therapeutic effects were evaluated by measuring ATP levels, neurosphere formation, 3D-invasion in collagen matrix, and viability. Protein expression profiles after drug treatment were evaluated by western blotting. In vivo anticancer efficacy of drugs was examined in a mouse orthotopic xenograft model.
Results
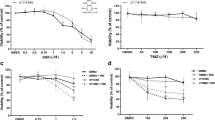
Combined treatment of GBM TSs with HCA or BCA and TMZ significantly reduced cell viability, stemness, and invasiveness. Expression levels of stemness-, invasiveness-, and mesenchymal transition-associated markers, Zeb1, N-cadherin, and β-catenin, were also substantially decreased by the combined treatment. The combined treatment also reduced tumor growth in a mouse orthotopic xenograft model.
Conclusion
Our findings suggest that HCA and BCA, combined with TMZ, are potential therapeutic agents in the treatment of GBM.





Similar content being viewed by others
References
Hoshide R, Jandial R (2016) 2016 world health organization classification of central nervous system tumors: an era of molecular biology. World Neurosurg 94:561–562. https://doi.org/10.1016/j.wneu.2016.07.082
Stupp R, Hegi ME, Mason WP, van den Bent MJ, Taphoorn MJ, Janzer RC, Ludwin SK, Allgeier A, Fisher B, Belanger K, Hau P, Brandes AA, Gijtenbeek J, Marosi C, Vecht CJ, Mokhtari K, Wesseling P, Villa S, Eisenhauer E, Gorlia T, Weller M, Lacombe D, Cairncross JG, Mirimanoff RO, European Organisation for R, Treatment of Cancer Brain T, Radiation Oncology G, National Cancer Institute of Canada Clinical Trials G (2009) Effects of radiotherapy with concomitant and adjuvant temozolomide versus radiotherapy alone on survival in glioblastoma in a randomised phase III study: 5-year analysis of the EORTC-NCIC trial. Lancet Oncol 10:459–466. https://doi.org/10.1016/S1470-2045(09)70025-7
Roh TH, Park HH, Kang SG, Moon JH, Kim EH, Hong CK, Ahn SS, Choi HJ, Cho J, Kim SH, Lee SK, Kim DS, Kim SH, Suh CO, Lee KS, Chang JH (2017) Long-term outcomes of concomitant chemoradiotherapy with temozolomide for newly diagnosed glioblastoma patients: a single-center analysis. Medicine (Baltimore) 96:e7422. https://doi.org/10.1097/MD.0000000000007422
Auffinger B, Spencer D, Pytel P, Ahmed AU, Lesniak MS (2015) The role of glioma stem cells in chemotherapy resistance and glioblastoma multiforme recurrence. Expert Rev Neurother 15:741–752. https://doi.org/10.1586/14737175.2015.1051968
Huber MA, Kraut N, Beug H (2005) Molecular requirements for epithelial-mesenchymal transition during tumor progression. Curr Opin Cell Biol 17:548–558. https://doi.org/10.1016/j.ceb.2005.08.001
Park J, Shim JK, Kang JH, Choi J, Chang JH, Kim SY, Kang SG (2018) Regulation of bioenergetics through dual inhibition of aldehyde dehydrogenase and mitochondrial complex I suppresses glioblastoma tumorspheres. Neuro Oncol 20:954–965. https://doi.org/10.1093/neuonc/nox243
Kim EH, Lee JH, Oh Y, Koh I, Shim JK, Park J, Choi J, Yun M, Jeon JY, Huh YM, Chang JH, Kim SH, Kim KS, Cheong JH, Kim P, Kang SG (2017) Inhibition of glioblastoma tumorspheres by combined treatment with 2-deoxyglucose and metformin. Neuro Oncol 19:197–207. https://doi.org/10.1093/neuonc/now174
Choi J, Lee JH, Koh I, Shim JK, Park J, Jeon JY, Yun M, Kim SH, Yook JI, Kim EH, Chang JH, Kim SH, Huh YM, Lee SJ, Pollak M, Kim P, Kang SG, Cheong JH (2016) Inhibiting stemness and invasive properties of glioblastoma tumorsphere by combined treatment with temozolomide and a newly designed biguanide (HL156A). Oncotarget 7:65643–65659. https://doi.org/10.18632/oncotarget.11595
Patrizii M, Bartucci M, Pine SR, Sabaawy HE (2018) Utility of glioblastoma patient-derived orthotopic xenografts in drug discovery and personalized therapy. Front Oncol 8:23. https://doi.org/10.3389/fonc.2018.00023
Kong BH, Park NR, Shim JK, Kim BK, Shin HJ, Lee JH, Huh YM, Lee SJ, Kim SH, Kim EH, Park EK, Chang JH, Kim DS, Kim SH, Hong YK, Kang SG, Lang FF (2013) Isolation of glioma cancer stem cells in relation to histological grades in glioma specimens. Childs Nerv Syst 29:217–229. https://doi.org/10.1007/s00381-012-1964-9
Kwak J, Shim JK, Kim DS, Lee JH, Choi J, Park J, Shin KJ, Kim SH, Kim P, Huh YM, Kim EH, Chang JH, Kim SH, Kang SG (2016) Isolation and characterization of tumorspheres from a recurrent pineoblastoma patient: feasibility of a patient-derived xenograft. Int J Oncol 49:569–578. https://doi.org/10.3892/ijo.2016.3554
Kim KM, Shim JK, Chang JH, Lee JH, Kim SH, Choi J, Park J, Kim EH, Kim SH, Huh YM, Lee SJ, Cheong JH, Kang SG (2016) Failure of a patient-derived xenograft for brain tumor model prepared by implantation of tissue fragments. Cancer Cell Int 16:43. https://doi.org/10.1186/s12935-016-0319-0
Kang SG, Cheong JH, Huh YM, Kim EH, Kim SH, Chang JH (2015) Potential use of glioblastoma tumorsphere: clinical credentialing. Arch Pharm Res 38:402–407. https://doi.org/10.1007/s12272-015-0564-0
Yoo KC, Suh Y, An Y, Lee HJ, Jeong YJ, Uddin N, Cui YH, Roh TH, Shim JK, Chang JH, Park JB, Kim MJ, Kim IG, Kang SG, Lee SJ (2018) Proinvasive extracellular matrix remodeling in tumor microenvironment in response to radiation. Oncogene 37:3317–3328. https://doi.org/10.1038/s41388-018-0199-y
Lim EJ, Suh Y, Yoo KC, Lee JH, Kim IG, Kim MJ, Chang JH, Kang SG, Lee SJ (2017) Tumor-associated mesenchymal stem-like cells provide extracellular signaling cue for invasiveness of glioblastoma cells. Oncotarget 8:1438–1448. https://doi.org/10.18632/oncotarget.13638
Eom H, Kaushik N, Yoo KC, Shim JK, Kwon M, Choi MY, Yoon T, Kang SG, Lee SJ (2018) MerTK mediates STAT3-KRAS/SRC-signaling axis for glioma stem cell maintenance. Artif Cells Nanomed Biotechnol. https://doi.org/10.1080/21691401.2018.1452022
Joseph JV, Conroy S, Pavlov K, Sontakke P, Tomar T, Eggens-Meijer E, Balasubramaniyan V, Wagemakers M, den Dunnen WF, Kruyt FA (2015) Hypoxia enhances migration and invasion in glioblastoma by promoting a mesenchymal shift mediated by the HIF1alpha-ZEB1 axis. Cancer Lett 359:107–116. https://doi.org/10.1016/j.canlet.2015.01.010
Monteiro AR, Hill R, Pilkington GJ, Madureira PA (2017) The role of hypoxia in glioblastoma invasion. Cells 6:45. https://doi.org/10.3390/cells6040045
Jackson M, Hassiotou F, Nowak A (2015) Glioblastoma stem-like cells: at the root of tumor recurrence and a therapeutic target. Carcinogenesis 36:177–185. https://doi.org/10.1093/carcin/bgu243
Iwadate Y (2016) Epithelial-mesenchymal transition in glioblastoma progression. Oncol Lett 11:1615–1620. https://doi.org/10.3892/ol.2016.4113
Hong SH, Ismail IA, Kang SM, Han DC, Kwon BM (2016) Cinnamaldehydes in cancer chemotherapy. Phytother Res 30:754–767. https://doi.org/10.1002/ptr.5592
Kwon B-M, Lee S-H, Cho Y-K, Bok S-H, So S-H, Youn M-R, Chang S-I (1997) Synthesis and biological activity of cinnamaldehydes as angiogenesis inhibitors. Bioorg Med Chem Lett 7:2473–2476. https://doi.org/10.1016/S0960-894X(97)10008-7
Lee K, Kwon BM, Kim K, Ryu J, Oh SJ, Lee KS, Kwon MG, Park SK, Kang JS, Lee CW, Kim HM (2009) Plasma pharmacokinetics and metabolism of the antitumour drug candidate 2′-benzoyloxycinnamaldehyde in rats. Xenobiotica 39:255–265. https://doi.org/10.1080/00498250802650069
Lee K, Park SK, Kwon BM, Kim K, Yu HE, Ryu J, Oh SJ, Lee KS, Kang JS, Lee CW, Kwon MG, Kim HM (2009) Transport and metabolism of the antitumour drug candidate 2′-benzoyloxycinnamaldehyde in Caco-2 cells. Xenobiotica 39:881–888. https://doi.org/10.3109/00498250903216000
Lee CW, Lee SH, Lee JW, Ban JO, Lee SY, Yoo HS, Jung JK, Moon DC, Oh KW, Hong JT (2007) 2-hydroxycinnamaldehyde inhibits SW620 colon cancer cell growth through AP-1 inactivation. J Pharmacol Sci 104:19–28
Ismail IA, Kang HS, Lee HJ, Chang H, Yun J, Lee CW, Kim NH, Kim HS, Yook JI, Hong SH, Kwon BM (2013) 2-Hydroxycinnamaldehyde inhibits the epithelial-mesenchymal transition in breast cancer cells. Breast Cancer Res Treat 137:697–708. https://doi.org/10.1007/s10549-012-2388-7
Lee SH, Lee SY, Son DJ, Lee H, Yoo HS, Song S, Oh KW, Han DC, Kwon BM, Hong JT (2005) Inhibitory effect of 2′-hydroxycinnamaldehyde on nitric oxide production through inhibition of NF-kappa B activation in RAW 264.7 cells. Biochem Pharmacol 69:791–799. https://doi.org/10.1016/j.bcp.2004.11.013
Hong SH, Kim J, Kim JM, Lee SY, Shin DS, Son KH, Han DC, Sung YK, Kwon BM (2007) Apoptosis induction of 2′-hydroxycinnamaldehyde as a proteasome inhibitor is associated with ER stress and mitochondrial perturbation in cancer cells. Biochem Pharmacol 74:557–565. https://doi.org/10.1016/j.bcp.2007.05.016
Kwak J, Shin HJ, Kim SH, Shim JK, Lee JH, Huh YM, Kim EH, Park EK, Chang JH, Kim SH, Hong YK, Kim DS, Lee SJ, Kang SG (2013) Isolation of tumor spheres and mesenchymal stem-like cells from a single primitive neuroectodermal tumor specimen. Childs Nerv Syst 29:2229–2239. https://doi.org/10.1007/s00381-013-2201-x
Chou TC (2010) Drug combination studies and their synergy quantification using the Chou-Talalay method. Cancer Res 70:440–446. https://doi.org/10.1158/0008-5472.CAN-09-1947
Lal S, Lacroix M, Tofilon P, Fuller GN, Sawaya R, Lang FF (2000) An implantable guide-screw system for brain tumor studies in small animals. J Neurosurg 92:326–333. https://doi.org/10.3171/jns.2000.92.2.0326
Junttila MR, de Sauvage FJ (2013) Influence of tumour micro-environment heterogeneity on therapeutic response. Nature 501:346–354. https://doi.org/10.1038/nature12626
Malanchi I, Santamaria-Martinez A, Susanto E, Peng H, Lehr HA, Delaloye JF, Huelsken J (2011) Interactions between cancer stem cells and their niche govern metastatic colonization. Nature 481:85–89. https://doi.org/10.1038/nature10694
Sasportas LS, Kasmieh R, Wakimoto H, Hingtgen S, van de Water JA, Mohapatra G, Figueiredo JL, Martuza RL, Weissleder R, Shah K (2009) Assessment of therapeutic efficacy and fate of engineered human mesenchymal stem cells for cancer therapy. Proc Natl Acad Sci USA 106:4822–4827. https://doi.org/10.1073/pnas.0806647106
Yoon SJ, Shim JK, Chang JH, Moon JH, Roh TH, Sung KS, Lee JH, Kim EH, Kim SH, Hong YK, Lee SJ, Huh YM, Kang SG (2016) Tumor mesenchymal stem-like cell as a prognostic marker in primary glioblastoma. Stem Cells Int 2016:6756983. https://doi.org/10.1155/2016/6756983
Lee CW, Hong DH, Han SB, Park SH, Kim HK, Kwon BM, Kim HM (1999) Inhibition of human tumor growth by 2′-hydroxy- and 2′-benzoyloxycinnamaldehydes. Planta Med 65:263–266. https://doi.org/10.1055/s-2006-960772
Shreaz S, Wani WA, Behbehani JM, Raja V, Irshad M, Karched M, Ali I, Siddiqi WA, Hun LT (2016) Cinnamaldehyde and its derivatives, a novel class of antifungal agents. Fitoterapia 112:116–131. https://doi.org/10.1016/j.fitote.2016.05.016
National Toxicology P (2004) NTP toxicology and carcinogenesis studies of trans-cinnamaldehyde (CAS No. 14371-10-9) in F344/N rats and B6C3F1 mice (feed studies). National Toxicology Program Technical Report Series, pp 1–281
Acknowledgements
This work was supported by grants from the Korean Health Technology R&D Project, Ministry of Health & Welfare, Republic of Korea (Grant No. HI17C2586), the Basic Science Research Program through the National Research Foundation of Korea funded by the MSIP: Ministry of Science, ICT and Future Planning, Republic of Korea (Grant No. NRF-2017M2A2A7A01071036).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All procedures performed in studies involving animals were in accordance with the ethical standards of the Yonsei University College of Medicine Institutional Animal Care and Use Committee. This article does not contain any studies involving human participants performed by any of the authors.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Jeong, H., Park, J., Shim, JK. et al. Combined treatment with 2′-hydroxycinnamaldehyde and temozolomide suppresses glioblastoma tumorspheres by decreasing stemness and invasiveness. J Neurooncol 143, 69–77 (2019). https://doi.org/10.1007/s11060-019-03151-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11060-019-03151-w