Abstract
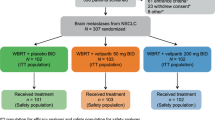
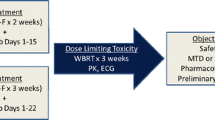
Perform a phase I study to evaluate the safety, and tolerability of vorinostat, an HDAC inhibitor, when combined with whole brain radiation treatment (WBRT) in patients with brain metastasis. A multi-institutional phase I clinical trial enrolled patients with a histological diagnosis of malignancy and radiographic evidence of brain metastasis. WBRT was 37.5 Gy in 2.5 Gy fractions delivered over 3 weeks. Vorinostat was administrated by mouth, once daily, Monday through Friday, concurrently with radiation treatment. The vorinostat dose was escalated from 200 to 400 mg daily using a 3+3 trial design. Seventeen patients were enrolled, 4 patients were excluded from the analysis due to either incorrect radiation dose (n = 1), or early treatment termination due to disease progression (n = 3). There were no treatment related grade 3 or higher toxicities in the 200 and 300 mg dose levels. In the 400 mg cohort there was a grade 3 pulmonary embolus and one death within 30 days of treatment. Both events were most likely related to disease progression rather than treatment; nonetheless, we conservatively classified the death as a dose limiting toxicity. We found Vorinostat administered with concurrent WBRT to be well tolerated to a dose of 300 mg once daily. This is the recommended dose for phase II study.

Similar content being viewed by others
References
Andrews DW (2008) Current neurosurgical management of brain metastases. Semin Oncol 35:100–107
Auperin A et al (1999) Prophylactic cranial irradiation for patients with small-cell lung cancer in complete remission. N Engl J Med 341:476–484
Baschnagel A et al (2009) Vorinostat enhances the radiosensitivity of a breast cancer brain metastatic cell line grown in vitro and as intracranial xenografts. Mol Cancer Ther 8:1589–1595
Bratland A et al (2011) Gastrointestinal toxicity of vorinostat: reanalysis of phase 1 study results with emphasis on dose-volume effects of pelvic radiotherapy. Radiat Oncol 6:33
Camphausen K et al (2005) Enhancement of in vitro and in vivo tumor cell radiosensitivity by valproic acid. Int J Cancer 114:380–386
Cerna D, Camphausen K, Tofilon PJ (2006) Histone deacetylation as a target for radiosensitization. Curr Top Dev Biol 73:173–204
Chang JE, Robins HI, Mehta MP (2007) Therapeutic advances in the treatment of brain metastases. Clin Adv Hematol Oncol 5:54–64
Chinnaiyan P et al (2012) Phase i trial of vorinostat combined with bevacizumab and cpt-11 in recurrent glioblastoma. Neuro Oncol 14:93–100
Chinnaiyan P et al (2005) Modulation of radiation response by histone deacetylase inhibition. Int J Radiat Oncol Biol Phys 62:223–229
Deorukhkar A et al (2010) Inhibition of radiation-induced DNA repair and prosurvival pathways contributes to vorinostat-mediated radiosensitization of pancreatic cancer cells. Pancreas 39:1277–1283
Duvic M et al (2007) Phase 2 trial of oral vorinostat (suberoylanilide hydroxamic acid, saha) for refractory cutaneous t-cell lymphoma (ctcl). Blood 109:31–39
Fakih MG et al (2012) A randomized phase ii study of two doses of vorinostat in combination with 5-fu/lv in patients with refractory colorectal cancer. Cancer Chemother Pharmacol 69:743–751
Friday BB et al (2012) Phase ii trial of vorinostat in combination with bortezomib in recurrent glioblastoma: a north central cancer treatment group study. Neuro Oncol 14:215–221
Galanis E et al (2009) Phase ii trial of vorinostat in recurrent glioblastoma multiforme: a north central cancer treatment group study. J Clin Oncol 27:2052–2058
Galanis E et al (2009) Phase ii trial of vorinostat in recurrent glioblastoma multiforme: a north central cancer treatment group study. J Clin Oncol 27:2052–2058
Gallinari P et al (2007) Hdacs, histone deacetylation and gene transcription: from molecular biology to cancer therapeutics. Cell Res 17:195–211
Gaspar L et al (1997) Recursive partitioning analysis (rpa) of prognostic factors in three radiation therapy oncology group (rtog) brain metastases trials. Int J Radiat Oncol Biol Phys 37:745–751
Hockly E et al (2003) Suberoylanilide hydroxamic acid, a histone deacetylase inhibitor, ameliorates motor deficits in a mouse model of huntington’s disease. Proc Natl Acad Sci USA 100:2041–2046
Ilker YE et al (2005) Suberoylanilide hydroxamic acid (saha) has potent anti-glioma properties in vitro, ex vivo and in vivo. J Neurochem 93:992–999
Karagiannis TC, El-Osta A (2006) Modulation of cellular radiation responses by histone deacetylase inhibitors. Oncogene 25:3885–3893
Kim MS et al (2003) Inhibition of histone deacetylase increases cytotoxicity to anticancer drugs targeting DNA. Cancer Res 63:7291–7300
Kim MS et al (2001) Histone deacetylases induce angiogenesis by negative regulation of tumor suppressor genes. Nat Med 7:437–443
Marks P et al (2001) Histone deacetylases and cancer: causes and therapies. Nat Rev 1:194–202
Marks PA et al (2004) Histone deacetylase inhibitors. Adv Cancer Res 91:137–168
Mueller S et al (2011) Cooperation of the hdac inhibitor vorinostat and radiation in metastatic neuroblastoma: efficacy and underlying mechanisms. Cancer Lett 306:223–229
Munshi A et al (2005) Histone deacetylase inhibitors radiosensitize human melanoma cells by suppressing DNA repair activity. Clin Cancer Res 11:4912–4922
Munshi A et al (2006) Vorinostat, a histone deacetylase inhibitor, enhances the response of human tumor cells to ionizing radiation through prolongation of gamma-h2ax foci. Mol Cancer Ther 5:1967–1974
Palmieri D et al (2009) Vorinostat inhibits brain metastatic colonization in a model of triple-negative breast cancer and induces DNA double-strand breaks. Clin Cancer Res 15:6148–6157
Patchell RA et al (1998) Postoperative radiotherapy in the treatment of single metastases to the brain: a randomized trial. JAMA 280:1485–1489
Patchell RA et al (1990) A randomized trial of surgery in the treatment of single metastases to the brain. N Engl J Med 322:494–500
Peereboom DM (2012) Clinical trial design in brain metastasis: approaches for a unique patient population. Curr Oncol Rep 14:91–96
Ramalingam SS et al (2010) Phase i study of vorinostat in patients with advanced solid tumors and hepatic dysfunction: a national cancer institute organ dysfunction working group study. J Clin Oncol 28:4507–4512
Ramaswamy B et al (2012) Phase i–ii study of vorinostat plus paclitaxel and bevacizumab in metastatic breast cancer: evidence for vorinostat-induced tubulin acetylation and hsp90 inhibition in vivo. Breast Cancer Res Treat 132:1063–1072
Ree AH et al (2010) Vorinostat, a histone deacetylase inhibitor, combined with pelvic palliative radiotherapy for gastrointestinal carcinoma: the pelvic radiation and vorinostat (pravo) phase 1 study. Lancet Oncol 11:459–464
Richon VM (2006) Cancer biology: mechanism of antitumour action of vorinostat (suberoylanilide hydroxamic acid), a novel histone deacetylase inhibitor. Br J Cancer 95:S2–S6
Richon VM et al (1998) A class of hybrid polar inducers of transformed cell differentiation inhibits histone deacetylases. Proc Natl Acad Sci USA 95:3003–3007
Schneider BJ et al (2012) Phase i study of vorinostat (suberoylanilide hydroxamic acid, nsc 701852) in combination with docetaxel in patients with advanced and relapsed solid malignancies. Invest New Drugs 30:249–257
Sperduto PW et al (2008) A new prognostic index and comparison to three other indices for patients with brain metastases: an analysis of 1,960 patients in the rtog database. Int J Radiat Oncol Biol Phys 70:510–514
Stathis A et al (2011) Phase i study of decitabine in combination with vorinostat in patients with advanced solid tumors and non-hodgkin’s lymphomas. Clin Cancer Res 17:1582–1590
Suh JH (2010) Stereotactic radiosurgery for the management of brain metastases. N Engl J Med 362:1119–1127
Ugur HC et al (2007) Continuous intracranial administration of suberoylanilide hydroxamic acid (saha) inhibits tumor growth in an orthotopic glioma model. J Neurooncol 83:267–275
Yin D et al (2007) Suberoylanilide hydroxamic acid, a histone deacetylase inhibitor: effects on gene expression and growth of glioma cells in vitro and in vivo. Clin Cancer Res 13:1045–1052
Zhang Y, Jung M, Dritschilo A (2004) Enhancement of radiation sensitivity of human squamous carcinoma cells by histone deacetylase inhibitors. Radiat Res 161:667–674
Acknowledgments
Merck & Co., Inc. supplied the Vorinostat in the preclinical and clinical studies and provided funding for both studies. Merck & Co., Inc. helped finance the clinical trial, the company also supplied the active drug, vorinostat.
Conflict of interest
Wenyin Shi: consultation for Varian, and Elekta. Hak Choy: advisory board for EMD and Bayer. Research grant from Celegene.
Author information
Authors and Affiliations
Corresponding author
Additional information
Wenyin Shi and Yaacov Richard Lawrence contributed equally to this project.
Rights and permissions
About this article
Cite this article
Shi, W., Lawrence, Y.R., Choy, H. et al. Vorinostat as a radiosensitizer for brain metastasis: a phase I clinical trial. J Neurooncol 118, 313–319 (2014). https://doi.org/10.1007/s11060-014-1433-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11060-014-1433-2