Abstract
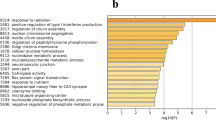
The hypothalamus plays a central role in controlling poultry reproductive activity. To increase our understanding of genes involved in egg laying of Huoyan geese, gene profiles in the hypothalamus of laying period and ceased period Huoyan geese were investigated using suppression subtractive hybridization (SSH) method. A total of 95 differentially expressed sequence tags (ESTs), including 46 up-regulated and 49 down-regulated sequences showed homology to known genes of the non-redundant NCBI databases. Bioinformatic analysis demonstrated that these genes were mainly involved in anatomical structure development, signal transduction, cellular nitrogen compound metabolic process, biosynthetic process, cellular protein modification process, cell differentiation, transport, cell adhesion, and reproduction. Ten ESTs were selected for further analyses by quantitative real-time RT-PCR (qRT-PCR). Whose most part of results were consistent with the SSH results. Of note, AdipoR2, Nrg1, and NCAM1, which related with secretion of GnRH and other hormones, were identified to be differentially expressed between laying period and ceased period. These findings provided a new source for mining genes related to higher laying performance of Huoyan geese, which facilitate our understanding of the reproductive biology of the goose.



Similar content being viewed by others
References
Xu Q, Zhao W, Chen Y, Tong Y, Rong G, Huang Z, Zhang Y, Chang G, Wu X, Chen G (2013) Transcriptome profiling of the goose (Anser cygnoides) ovaries identify laying and broodiness phenotypes. PLoS One 8(2):e55496. doi:10.1371/journal.pone.0055496
Padmanabhan V, Karsch FJ, Lee JS (2002) Hypothalamic, pituitary and gonadal regulation of FSH. Reprod Suppl 59:67–82
Yoshimura T (2006) Molecular mechanism of the photoperiodic response of gonads in birds and mammals. Comp Biochem Physiol A 144(3):345–350. doi:10.1016/j.cbpa.2005.09.009
Sharp PJ (1993) Photoperiodic control of reproduction in the domestic hen. Poult Sci 72(5):897–905
Onagbesan O, Metayer S, Tona K, Williams J, Decuypere E, Bruggeman V (2006) Effects of genotype and feed allowance on plasma luteinizing hormones, follicle-stimulating hormones, progesterone, estradiol levels, follicle differentiation, and egg production rates of broiler breeder hens. Poult Sci 85(7):1245–1258
Sharp PJ (2005) Photoperiodic regulation of seasonal breeding in birds. Ann N Y Acad Sci 1040:189–199. doi:10.1196/annals.1327.024
Tsutsui K, Bentley GE, Ubuka T, Saigoh E, Yin H, Osugi T, Inoue K, Chowdhury VS, Ukena K, Ciccone N, Sharp PJ, Wingfield JC (2007) The general and comparative biology of gonadotropin-inhibitory hormone (GnIH). Gen Comp Endocrinol 153(1–3):365–370. doi:10.1016/j.ygcen.2006.10.005
Dunn I, Miao Y, Morris A, Romanov M, Wilson P, Waddington D (2003) A study of association between genetic markers in candidate genes and reproductive traits in one generation of a commercial broiler breeder hen population. Heredity 92(2):128–134
Chaiseha Y, Youngren OM, El Halawani ME (2004) Expression of vasoactive intestinal peptide receptor messenger RNA in the hypothalamus and pituitary throughout the turkey reproductive cycle. Biol Reprod 70(3):593–599. doi:10.1095/biolreprod.103.022715
Etches R, Petitte J, Anderson-Langmuir C (1984) Interrelationships between the hypothalamus, pituitary gland, ovary, adrenal gland, and the open period for LH release in the hen (Gallus domesticus). J Exp Zool 232(3):501–511
Shen X, Zeng H, Xie L, He J, Li J, Xie X, Luo C, Xu H, Zhou M, Nie Q (2012) The GTPase activating Rap/RanGAP domain-Like 1 gene is associated with chicken reproductive traits. PLoS One 7(4):e33851
Shiue YL, Chen LR, Chen CF, Chen YL, Ju JP, Chao CH, Lin YP, Kuo YM, Tang PC, Lee YP (2006) Identification of transcripts related to high egg production in the chicken hypothalamus and pituitary gland. Theriogenology 66(5):1274–1283. doi:10.1016/j.theriogenology.2006.03.037
Kang B, Guo JR, Yang HM, Zhou RJ, Liu JX, Li SZ, Dong CY (2009) Differential expression profiling of ovarian genes in prelaying and laying geese. Poult Sci 88(9):1975–1983. doi:10.3382/ps.2008-00519
Yen CF, Lin HW, Hsu JC, Lin C, Shen TF, Ding ST (2006) The expression of pituitary gland genes in laying geese. Poult Sci 85(12):2265–2269
McCarthy FM, Bridges SM, Wang N, Magee GB, Williams WP, Luthe DS, Burgess SC (2007) AgBase: a unified resource for functional analysis in agriculture. Nucleic Acids Res 35:D599–603. doi:10.1093/nar/gkl936
Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2(−Delta Delta C(T)) Method. Methods 25(4):402–408. doi:10.1006/meth.2001.1262
Kiezun M, Maleszka A, Smolinska N, Nitkiewicz A, Kaminski T (2013) Expression of adiponectin receptors 1 (AdipoR1) and 2 (AdipoR2) in the porcine pituitary during the oestrous cycle. Reprod Biol Endocrinol 11:18. doi:10.1186/1477-7827-11-18
Takemura Y, Osuga Y, Yamauchi T, Kobayashi M, Harada M, Hirata T, Morimoto C, Hirota Y, Yoshino O, Koga K, Yano T, Kadowaki T, Taketani Y (2006) Expression of adiponectin receptors and its possible implication in the human endometrium. Endocrinology 147(7):3203–3210. doi:10.1210/en.2005-1510
Prevot V, Rio C, Cho GJ, Lomniczi A, Heger S, Neville CM, Rosenthal NA, Ojeda SR, Corfas G (2003) Normal female sexual development requires neuregulin-erbB receptor signaling in hypothalamic astrocytes. J Neurosci 23(1):230–239
Zhao W, Shen Y, Ren S (2011) Endogenous expression of Neuregulin-1 (Nrg1) as a potential modulator of prolactin (PRL) secretion in GH3 cells. Cell Tissue Res 344(2):313–320
Rubinek T, Yu R, Hadani M, Barkai G, Nass D, Melmed S, Shimon I (2003) The cell adhesion molecules N-cadherin and neural cell adhesion molecule regulate human growth hormone: a novel mechanism for regulating pituitary hormone secretion. J Clin Endocrinol Metab 88(8):3724–3730
Kanasaki H, Purwana I, Oride A, Mijiddorj T, Miyazaki K (2011) Extracellular signal-regulated kinase (ERK) activation and mitogen-activated protein kinase phosphatase 1 induction by pulsatile gonadotropin-releasing hormone in pituitary Gonadotrophs. J Signal Transduct 2012:198527
Bliss SP, Navratil AM, Xie J, Roberson MS (2010) GnRH signaling, the gonadotrope and endocrine control of fertility. Front Neuroendocrinol 31(3):322–340. doi:10.1016/j.yfrne.2010.04.002
Shimizu F, Sanada K, Fukada Y (2003) Purification and immunohistochemical analysis of calcium-binding proteins expressed in the chick pineal gland. J Pineal Res 34(3):208–216
Waki H, Nakamura M, Yamauchi T, Wakabayashi K-I, Yu J, Hirose-Yotsuya L, Take K, Sun W, Iwabu M, Okada-Iwabu M (2011) Global mapping of cell type–specific open chromatin by FAIRE-seq reveals the regulatory role of the NFI family in adipocyte differentiation. PLoS Genet 7(10):e1002311
Brann DW, Mahesh VB (1997) Excitatory amino acids: evidence for a role in the control of reproduction and anterior pituitary hormone secretion. Endocr Rev 18(5):678–700
Worley PF, Baraban JM, Van Dop C, Neer EJ, Snyder SH (1986) Go, a guanine nucleotide-binding protein: immunohistochemical localization in rat brain resembles distribution of second messenger systems. Proc Natl Acad Sci 83(12):4561–4565
Diatchenko L, Lau YF, Campbell AP, Chenchik A, Moqadam F, Huang B, Lukyanov S, Lukyanov K, Gurskaya N, Sverdlov ED, Siebert PD (1996) Suppression subtractive hybridization: a method for generating differentially regulated or tissue-specific cDNA probes and libraries. Proc Natl Acad Sci USA 93(12):6025–6030
Tabandeh MR, Hosseini A, Saeb M, Kafi M, Saeb S (2010) Changes in the gene expression of adiponectin and adiponectin receptors (AdipoR1 and AdipoR2) in ovarian follicular cells of dairy cow at different stages of development. Theriogenology 73(5):659–669. doi:10.1016/j.theriogenology.2009.11.006
Mitchell M, Armstrong DT, Robker RL, Norman RJ (2005) Adipokines: implications for female fertility and obesity. Reproduction 130(5):583–597. doi:10.1530/rep.1.00521
Michalakis KG, Segars JH (2010) The role of adiponectin in reproduction: from polycystic ovary syndrome to assisted reproduction. Fertil Steril 94(6):1949–1957. doi:10.1016/j.fertnstert.2010.05.010
Kubota N, Yano W, Kubota T, Yamauchi T, Itoh S, Kumagai H, Kozono H, Takamoto I, Okamoto S, Shiuchi T, Suzuki R, Satoh H, Tsuchida A, Moroi M, Sugi K, Noda T, Ebinuma H, Ueta Y, Kondo T, Araki E, Ezaki O, Nagai R, Tobe K, Terauchi Y, Ueki K, Minokoshi Y, Kadowaki T (2007) Adiponectin stimulates AMP-activated protein kinase in the hypothalamus and increases food intake. Cell Metab 6(1):55–68. doi:10.1016/j.cmet.2007.06.003
Wen JP, Lv WS, Yang J, Nie AF, Cheng XB, Yang Y, Ge Y, Li XY, Ning G (2008) Globular adiponectin inhibits GnRH secretion from GT1-7 hypothalamic GnRH neurons by induction of hyperpolarization of membrane potential. Biochem Biophys Res Commun 371(4):756–761. doi:10.1016/j.bbrc.2008.04.146
Rodriguez-Pacheco F, Martinez-Fuentes AJ, Tovar S, Pinilla L, Tena-Sempere M, Dieguez C, Castano JP, Malagon MM (2007) Regulation of pituitary cell function by adiponectin. Endocrinology 148(1):401–410. doi:10.1210/en.2006-1019
Lu M, Tang Q, Olefsky JM, Mellon PL, Webster NJ (2008) Adiponectin activates adenosine monophosphate-activated protein kinase and decreases luteinizing hormone secretion in LbetaT2 gonadotropes. Mol Endocrinol 22(3):760–771. doi:10.1210/me.2007-0330
Hoyda TD, Fry M, Ahima RS, Ferguson AV (2007) Adiponectin selectively inhibits oxytocin neurons of the paraventricular nucleus of the hypothalamus. J Physiol 585(Pt 3):805–816. doi:10.1113/jphysiol.2007.144519
Klein I, Sanchez-Alavez M, Tabarean I, Schaefer J, Holmberg KH, Klaus J, Xia F, Marcondes MC, Dubins JS, Morrison B, Zhukov V, Sanchez-Gonzalez A, Mitsukawa K, Hadcock JR, Bartfai T, Conti B (2011) AdipoR1 and 2 are expressed on warm sensitive neurons of the hypothalamic preoptic area and contribute to central hyperthermic effects of adiponectin. Brain Res 1423:1–9. doi:10.1016/j.brainres.2011.09.019
Orr-Urtreger A, Trakhtenbrot L, Ben-Levy R, Wen D, Rechavi G, Lonai P, Yarden Y (1993) Neural expression and chromosomal mapping of Neu differentiation factor to 8p12-p21. Proc Natl Acad Sci U S A 90(5):1867–1871
Jacobsen NE, Abadi N, Sliwkowski MX, Reilly D, Skelton NJ, Fairbrother WJ (1996) High-resolution solution structure of the EGF-like domain of heregulin-α. Biochemistry 35(11):3402–3417
Corfas G, Rosen KM, Aratake H, Krauss R, Fischbach GD (1995) Differential expression of ARIA isoforms in the rat brain. Neuron 14(1):103–115 0896-6273(95)90244-9 [pii]
Bernstein HG, Lendeckel U, Bertram I, Bukowska A, Kanakis D, Dobrowolny H, Stauch R, Krell D, Mawrin C, Budinger E, Keilhoff G, Bogerts B (2006) Localization of neuregulin-1alpha (heregulin-alpha) and one of its receptors, ErbB-4 tyrosine kinase, in developing and adult human brain. Brain Res Bull 69(5):546–559. doi:10.1016/j.brainresbull.2006.02.017
Zhao W, Ren SG (2011) Neuregulin-1 (Nrg1) is mainly expressed in rat pituitary gonadotroph cells and possibly regulates prolactin (PRL) secretion in a juxtacrine manner. J Neuroendocrinol 23(12):1252–1262
Kansaku N, Ohkubo T, Okabayashi H, Guemene D, Kuhnlein U, Zadworny D, Shimada K (2005) Cloning of duck PRL cDNA and genomic DNA. Gen Comp Endocrinol 141(1):39–47. doi:10.1016/j.ygcen.2004.11.017
Wong EA, Ferrin NH, Silsby JL, el Halawani ME (1991) Cloning of a turkey prolactin cDNA: expression of prolactin mRNA throughout the reproductive cycle of the domestic turkey (Meleagris gallopavo). Gen Comp Endocrinol 83(1):18–26
Bonfanti L (2006) PSA-NCAM in mammalian structural plasticity and neurogenesis. Prog Neurobiol 80(3):129–164. doi:10.1016/j.pneurobio.2006.08.003
Perera AD, Lagenaur CF, Plant TM (1993) Postnatal expression of polysialic acid-neural cell adhesion molecule in the hypothalamus of the male rhesus monkey (Macaca mulatta). Endocrinology 133(6):2729–2735
Parkash J, Kaur G (2005) Neuronal-glial plasticity in gonadotropin-releasing hormone release in adult female rats: role of the polysialylated form of the neural cell adhesion molecule. J Endocrinol 186(2):397–409. doi:10.1677/joe.1.06156
Langley OK, Aletsee-Ufrecht MC, Grant NJ, Gratzl M (1989) Expression of the neural cell adhesion molecule NCAM in endocrine cells. J Histochem Cytochem 37(6):781–791
Edelman GM, Crossin KL (1991) Cell adhesion molecules: implications for a molecular histology. Annu Rev Biochem 60:155–190. doi:10.1146/annurev.bi.60.070191.001103
Ramsdell JS, Tashjian AH Jr (1989) GH4 pituitary cell variants selected as nonresponsive to thyrotropin-releasing hormone-enhanced substratum adhesion are nonresponsive to epidermal growth factor: evidence for a common signaling defect. J Cell Physiol 141(3):565–572. doi:10.1002/jcp.1041410315
Hull KL, Harvey S (2001) Growth hormone: roles in female reproduction. J Endocrinol 168(1):1–23
Chen C-F, Shiue Y-L, Yen C-J, Tang P-C, Chang H-C, Lee Y-P (2007) Laying traits and underlying transcripts, expressed in the hypothalamus and pituitary gland, that were associated with egg production variability in chickens. Theriogenology 68(9):1305–1315
Acknowledgments
This study was supported by the National Natural Science Foundation of China (Grant No. 31172286). We would like to thank the staff of Panjin Jiyuan goose breeding farm, who assisted in the collection of goose hypothalamus samples.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Luan, X., Cao, Z., Li, R. et al. Differential expression profiling of hypothalamus genes in laying period and ceased period Huoyan geese. Mol Biol Rep 41, 3401–3411 (2014). https://doi.org/10.1007/s11033-014-3202-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11033-014-3202-x