Abstract
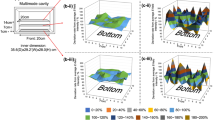
The heating behavior of silicon carbide reaction platforms under 2.45 GHz microwave irradiation was investigated with the aid of online thermoimaging cameras and multiple-channel fiber-optic probe temperature sensors placed inside the wells/vials of the silicon carbide microtiter plates. Microwave irradiation leads to a rapid and homogeneous heating of the entire plate, with minimal deviations in the temperature recorded at different positions of the plate or inside the wells. In temperature-controlled experiments using dedicated multimode reactors, solvents with different microwave absorption characteristics can be heated in parallel in individual wells/vials of the silicon carbide plate reaching the same set temperature. Due to the large heat capacity and high thermal conductivity of silicon carbide, the plates are able to moderate any field inhomogeneities inside a microwave cavity. Although the heating of the plates can be performed extremely efficiently inside a microwave reactor, heating and synthetic applications can alternatively be carried out by applying conventional conductive heating of the silicon carbide plates on a standard hotplate. Due to the slower heating of the silicon carbide material under these conditions, somewhat longer reaction times will be required.
Similar content being viewed by others
References
Alcázar J (2005) Reproducibility across microwave instruments: preparation of a set of 24 compounds on a multiwell plate under temperature-controlled conditions. J Comb Chem 7: 353–355. doi:10.1021/cc049815k
Baeraky TA (2002) Microwave measurements of the dielectric properties of silicon carbide at high temperature. Egypt J Sol 25: 263–273
Baghbanzadeh M, Molnar M, Damm M, Reidlinger C, Dabiri M, Kappe CO (2009) Parallel microwave synthesis of 2-styrylquinazolin-4(3H)-ones in a high-throughput platform using HPLC/GC vials as reaction vessels. J Comb Chem 11: (in press)
Carvalho AP, Malcata FX (2005) Preparation of fatty acid methyl esters for gas-chromatographic analysis of marine lipids: insight studies. J Agric Food Chem 53: 5049–5059. doi:10.1021/jf048788i
Choyke, WJ, Matsunami, H, Pensl, G (eds) (2004) Silicon carbide: recent major advances. Springer, Berlin
Damm M, Kappe CO (2009) High-throughput experimentation platform: parallel microwave chemistry in HPLC/GC vials. J Comb Chem 11: 460–468. doi:10.1021/cc900007w
Damm M, Rechberger G, Kollroser M, Kappe CO (2009) An evaluation of microwave-assisted derivatization procedures using hyphenated mass spectrometric techniques. J Chrom A (in press)
Dimitrakis GA, National Centre for Industrial Microwave Processing (NCIMP), University of Nottingham (unpublished results).
Gupta, M, Wong Wei Leong, E (eds) (2007) Microwaves and metals. J. Wiley & Sons, Asia
Harris GL (ed) (1995) Properties of silicon carbide. Institute of Electrical Engineers
Herrero MA, Kremsner JM, Kappe CO (2008) Nonthermal microwave effects revisited: on the importance of internal temperature monitoring and agitation in microwave chemistry. J Org Chem 73: 36–47. doi:10.1021/jo7022697
Horikoshi S, Hamamura T, Kajitani M, Yoshizawa-Fujita M, Serpone N (2008) Green chemistry with a novel 5.8-GHz microwave apparatus. Prompt one-pot solvent-free synthesis of a major ionic liquid: the 1-butyl-3-methylimidazolium tetrafluoroborate system. Org Process Res Dev 12: 1089–1093
Igarashi M, Tsuzuki T, Kambe T, Miyazawa T (2004) Recommended methods of fatty acid methylester preparation for conjugated dienes and trienes in food and biological samples. J Nutr Sci Vitaminol (Tokyo) 50: 121–128
Kappe CO (2004) Controlled microwave heating in modern organic synthesis. Angew Chem Int Ed 43: 6250–6284. doi:10.1002/anie.200400655
Kappe CO, Dallinger D (2009) Controlled microwave heating in modern organic synthesis. Highlights from the 2004–2008 literature. Mol Divers 13: 71–193. doi:10.1007/s11030-009-9138-8
Kappe CO, Stadler A (2001) Automated library generation using sequential microwave-assisted chemistry. Application toward the Biginelli multicomponent condensation. J Comb Chem 3: 624–630. doi:10.1021/cc010044j
Kappe CO, Dallinger D, Murphree SS (2009) Practical microwave synthesis for organic chemists—strategies, instruments, and protocols. Wiley-VCH, Weinheim
Karstädt D, Möllmann KP, Vollmer M (2004) Eier im Wellensalat: Experimente mit der Haushaltsmikrowelle. Phys Unserer Zeit 35: 90–96. doi:10.1002/piuz.200401033
Koppitz M (2008) Maximizing efficiency in the production of compound libraries. J Comb Chem 10: 573–579. doi:10.1021/cc800004a
Kremsner JM, Kappe CO (2006) Silicon carbide passive heating elements in microwave-assisted organic synthesis. J Org Chem 71: 4651–4658. doi:10.1021/jo060692v and references cited therein
Kremsner JM, Stadler A, Kappe CO (2007) High-throughput microwave-assisted organic synthesis: moving from automated sequential to parallel library-generation formats in silicon carbide microtiter plates. J Comb Chem 9: 285–291. doi:10.1021/cc060138z
Macleod C, Martinez-Teipel BI, Barker WM, Dolle RE (2006) Annulation of primary amines to piperazines and diazaspirocycles utilizing α-methyl benzyl resin. J Comb Chem 8: 132–140. doi:10.1021/cc050106w
Martinez-Teipel B, Green RC, Dolle RE (2004) Microwave-assisted synthesis of di- and trisubstituted ureas from thiophenoxy carbamate resins. QSAR Comb Sci 23: 854–858. doi:10.1002/qsar.200420043
Matloobi M, Kappe CO (2007) Parallel processing of microwave-assisted organic transformations. Comb Chem High Throughput Screen 10: 735–750. doi:10.2174/138620707783018496
Medina I, Aubourg S, Gallardo JM, Perezmartin R (1992) Comparison of six methylation methods for analysis of the fatty acid composition of albacore lipid. Int J Food Sci Technol 27: 597–601
Nüchter M, Ondruschka B (2003) Tools for microwave-assisted parallel synthesis and combinatorial chemistry. Mol Divers 7: 253–264. doi:10.1023/B:MODI.0000006916.69862.3d
Peters FT, Drvarov O, Lottner S, Spellmeier A, Rieger K, Haefeli WE, Maurer HH (2009) A systematic comparison of four different workup procedures for systematic toxicological analysis of urine samples using gas chromatography-mass spectrometry. Anal Bioanal Chem 393: 735–745. doi:10.1007/s00216-008-2471-4
Pfleger, K, Weber, A, Maurer, HH (eds) (2007) Mass spectral and GC data of drugs, poisons, pesticides, pollutants and their metabolites, 3rd edn. Wiley-VCH, Weinheim
Saddow, SE, Agarwal, A (eds) (2004) Advances in silicon carbide processing and applications. Artech House Inc, Norwood, MA
Segura J, Ventura R, Jurado C (1998) Derivatization procedures for gas chromatographic-mass spectrometric determination of xenobiotics in biological samples, with special attention to drugs of abuse and doping agents. J Chromatogr B 713: 61–90. doi:10.1016/S0378-4347(98)00089-9
Stadler A, Yousefi BH, Dallinger D, Walla P, Vander Eycken E, Kaval N, Kappe CO (2003) Scalability of microwave-assisted organic synthesis. From single-mode to multimode parallel batch reactors. Org Process Res Dev 7: 707–716. doi:10.1021/op034075+
Treu M, Karner T, Kousek R, Berger H, Mayer M, McConnell DB, Stadler A (2008) Microwave-assisted parallel synthesis of fused heterocycles in a novel parallel multimode reactor. J Comb Chem 10: 863–868. doi:10.1021/cc800081b
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Rights and permissions
About this article
Cite this article
Damm, M., Kappe, C.O. Parallel microwave chemistry in silicon carbide reactor platforms: an in-depth investigation into heating characteristics. Mol Divers 13, 529–543 (2009). https://doi.org/10.1007/s11030-009-9167-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11030-009-9167-3