Abstract
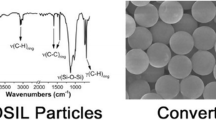
The continuous wet chemical preparation of micro- and nanoparticles is a major challenge for the large-scale production of functional colloids. Here we present a general synthetic strategy for sol–gel-based materials via an additive free homogenous approach avoiding emulsion-based systems. A variety of different silica and organically-modified silica (ORMOSIL) spherical particles were prepared applying a condensation of prehydrolyzed alcoholic solution of organotrialkoxysilanes in a microjet reactor. This method presents a unique wet chemical production method for nano- and microscale materials. Methyl-, ethyl-, propyl-, vinyl-, phenyl-, and mixed ORMOSIL particles in the range of 75 nm–2 µm were successfully synthesized without the addition of stabilizing surfactants. The method was also investigated for the continuous preparation of pure silica particles, and we succeeded to produce continuously up to 23 g particles per minute. The influence of different organic groups on the crosslinking of the siloxane network was systematically studied applying various spectroscopic and thermoanalytical methods. The degree of condensation of the obtained particles depends on the organic rests of the trialkoxysilanes, which was studied with 29Si CP-MAS NMR. We were able to show that phenyl silsesquioxanes show less condensation in the particles than smaller alkyl or vinyl groups. In addition, the silane concentration has a significant influence on the particle size. Generally, smaller particle diameters are obtained after decreasing the silane concentration. The described process delivers a fast and large scale wet chemical production of various silsesquioxane and silica particles without the use of additives and is therefore suited for a variety of potential applications where high purity of the particles is necessary.







Similar content being viewed by others
Change history
20 January 2020
An amendment to this paper has been published and can be accessed via a link at the top of the paper.
20 January 2020
An amendment to this paper has been published and can be accessed via a link at the top of the paper.
References
Pachón LD, Rothenberg G (2008) Transition-metal nanoparticles: synthesis, stability and the leaching issue. Appl Organomet Chem 22(6):288–299. https://doi.org/10.1002/aoc.1382
Wang L, Wang L, Xia T, Dong L, Bian G, Chen H (2004) Direct fluorescence quantification of chromium(VI) in wastewater with organic nanoparticles sensor. Anal Sci 20(7):1013–1017
Barbé CJ, Arendse F, Comte P, Jirousek M, Lenzmann F, Shklover V, Grätzel M (1997) Nanocrystalline titanium oxide electrodes for photovoltaic applications. J Am Ceram Soc 80(12):3157–3171
Ito A, Shinkai M, Honda H, Kobayashi T (2005) Medical application of functionalized magnetic nanoparticles. J Biosci Bioeng 100(1):1–11. https://doi.org/10.1263/jbb.100.1
Mihindukulasuriya SDF, Lim L-T (2014) Nanotechnology development in food packaging: a review. Trends Food Sci Tech 40(2):149–167. https://doi.org/10.1016/j.tifs.2014.09.009
Becheri A, Dürr M, Lo Nostro P, Baglioni P (2008) Synthesis and characterization of zinc oxide nanoparticles: application to textiles as UV-absorbers. J Nanopart Res 10(4):679–689. https://doi.org/10.1007/s11051-007-9318-3
Rahman MT, Rebrov EV (2014) Microreactors for gold nanoparticles synthesis: from Faraday to flow. Processes 2(2):466–493. https://doi.org/10.3390/pr2020466
Khan SA, Günther A, Schmidt MA, Jensen KF (2004) Microfluidic synthesis of colloidal silica. Langmuir 20(20):8604–8611. https://doi.org/10.1021/la0499012
Gutsch A, Krämer M, Michael G, Mühlenweg H, Pridöhl M, Zimmermann G (2002) Gas-phase production of nanoparticles. KONA 20:24–37
Suh YK, Kang S (2010) A review on mixing in microfluidics. Micromachines 1(3):82–111. https://doi.org/10.3390/mi1030082
Marre S, Jensen KF (2010) Synthesis of micro and nanostructures in microfluidic systems. Chem Soc Rev 39(3):1183–1202. https://doi.org/10.1039/b821324k
Zhao C-X, He L, Qiao SZ, Middelberg APJ (2011) Nanoparticle synthesis in microreactors. Chem Eng Sci 66(7):1463–1479. https://doi.org/10.1016/j.ces.2010.08.039
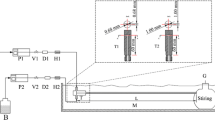
Betke A, Kickelbick G (2014) Bottom-up, wet chemical technique for the continuous synthesis of inorganic nanoparticles. Inorganics 2(1):1–15. https://doi.org/10.3390/inorganics2010001
Penth B (2007) Kontinuierliche Produktion in Mikroreaktoren. German Patent DE102006004350 A1.
Gutierrez L, Gomez L, Irusta S, Arruebo M, Santamaria J (2011) Comparative study of the synthesis of silica nanoparticles in micromixer–microreactor and batch reactor systems. Chem Eng J 171(2):674–683. https://doi.org/10.1016/j.cej.2011.05.019
Su M, Su H, Du B, Li X, Ren G, Wang S (2014) The properties of silica nanoparticles with high monodispersity synthesized in the microreactor system. J Sol-Gel Sci Technol 72(2):375–384. https://doi.org/10.1007/s10971-014-3445-y
Kessler D, Löwe H, Theato P (2009) Synthesis of defined poly(silsesquioxane)s: fast polycondensation of trialkoxysilanes in a continuous-flow microreactor. Macromol Chem Phys 210(10):807–813. https://doi.org/10.1002/macp.200800611
Stöber W, Fink A, Bohn E (1968) Controlled growth of monodisperse silica spheres in the micron size range. J Colloid Interface Sci 26:62–69
Van Blaaderen A, Vrij A (1993) Synthesis and characterization of monodisperse colloidal organo-silica spheres. J Colloid Interface Sci 156:1–18
Etienne M, Lebeau B, Walcarius A (2002) Organically-modified mesoporous silica spheres with MCM-41 architecture. New J Chem 26(4):384–386. https://doi.org/10.1039/b110741k
Arkhireeva A, Hay JN (2003) Synthesis of sub-200 nm silsesquioxane particles using a modified Stöber sol–gel route. J Mater Chem 13(12):3122–3127. https://doi.org/10.1039/b306994j
Choi JY, Kim CH, Kim DK (1998) Formation and characterization of monodisperse, spherical organo-silica powders from organo-alkoxysilane-water system. J Am Ceram Soc 81(5):1184–1188
Ottenbrite RM, Wall JS (2000) Self-catalyzed synthesis of organo-silica nanoparticles. J Am Ceram Soc 83(12):3214–3215
Boday DJ, Tolbert S, Keller MW, Li Z, Wertz JT, Muriithi B, Loy DA (2014) Non-hydrolytic formation of silica and polysilsesquioxane particles from alkoxysilane monomers with formic acid in toluene/tetrahydrofuran solutions. J Nanopart Res 16(3):1–13. https://doi.org/10.1007/s11051-014-2313-6
Krüner B, Odenwald C, Tolosa A, Schreiber A, Aslan M, Kickelbick G, Presser V (2017) Carbide-derived carbon beads with tunable nanopores from continuously produced polysilsesquioxanes for supercapacitor electrodes. Sustain Energy Fuels 1:1588–1600. https://doi.org/10.1039/C7SE00265C10.1039/c7se00265c
Schneider CA, Rasband WS, Eliceiri KW (2012) NIH Image to ImageJ: 25 years of image analysis. Nat Methods 9(7):671–675. https://doi.org/10.1038/nmeth.2089
Bah T (2011) Inkscape: guide to a vector drawing program, 4th edn.
Fournier M-C, Falk L, Villermaux J (1996) A new parallel competing reaction system for assessing micromixing efficiency - experimental approach. Chem Eng Sci 51(22):5053–5064
Dushman S (1904) The rate of the reaction between iodic and hydriodic acids. J Phys Chem 8(7):453–482. https://doi.org/10.1021/j150061a001
Commenge J-M, Falk L (2011) Villermaux–Dushman protocol for experimental characterization of micromixers. Chem Eng Process 50(10):979–990. https://doi.org/10.1016/j.cep.2011.06.006
Brinker CJ, Tallant DR, Roth EP, Ashley CS (1986) Sol-gel transition in simple silicates III. Structural studies during densification. J Non-Cryst Solids 82:117–126
Brinker CJ (1988) Hydrolysis and condensation of silicates: effects on structure. J Non-Cryst Solids 100:31–50
Klein LC, Jitianu A (2010) Organic–inorganic hybrid melting gels. J Sol-Gel Sci Technol 55(1):86–93. https://doi.org/10.1007/s10971-010-2219-4
Brochier Salon M-C, Bayle P-A, Abdelmouleh M, Boufi S, Belgacem MN (2008) Kinetics of hydrolysis and self condensation reactions of silanes by NMR spectroscopy. Colloids Surf A: Physicochem Eng Asp 312(2-3):83–91. https://doi.org/10.1016/j.colsurfa.2007.06.028
Brochier Salon M-C, Belgacem MN (2010) Competition between hydrolysis and condensation reactions of trialkoxysilanes, as a function of the amount of water and the nature of the organic group. Colloids Surf A: Physicochem Eng Asp 366(1–3):147–154. https://doi.org/10.1016/j.colsurfa.2010.06.002
Rao AV, Kulkarni MM, Amalnerkar DP, Seth T (2003) Superhydrophobic silica aerogels based on methyltrimethoxysilane precursor. J Non-Cryst Solids 330:187–195. https://doi.org/10.1016/j.jnoncrysol.2003.08.048
Sharma RK, Das S, Maitra A (2004) Surface modified ormosil nanoparticles. J Colloid Interface Sci 277(2):342–346. https://doi.org/10.1016/j.jcis.2004.04.019
Macan J, Tadanaga K, Tatsumisago M (2010) Influence of copolymerization with alkyltrialkoxysilanes on condensation and thermal behaviour of poly(phenylsilsesquioxane) particles. J Sol-Gel Sci Technol 53(1):31–37. https://doi.org/10.1007/s10971-009-2051-x
Jesson DA, Abel M-L, Hay JN, Smith PA, Watts JF (2006) Organic-inorganic hybrid nanoparticles: Surface characteristics and interactions with a polyester resin. Langmuir 22(11):5144–5151
Launer PJ (1987) Infrared analysis of organosilicon compounds: Spectra-structure correlations. In Anderson R, Arkles B, Larson GL (eds) Silicone Compounds Register and Review, 4th edn, pp 100–103
Nam K-H, Lee T-H, Bae B-S, Popall M (2006) Condensation reaction of 3-(methacryloxypropyl)-trimethoxysilane and diisobutylsilanediol in non-hydrolytic sol-gel process. J Sol-Gel Sci Technol 39(3):255–260. https://doi.org/10.1007/s10971-006-7884-y
Komori Y, Nakashima H, Hayashi S, Sugahara Y (2005) Silicon-29 cross-polarization/magic-angle-spinning NMR study of inorganic–organic hybrids: homogeneity of sol–gel derived hybrid gels. J Non-Cryst Solids 351(2):97–103. https://doi.org/10.1016/j.jnoncrysol.2004.10.005
Loy DA, Jamison GM, Baugher BM, Russick EM, Assink RA, Prabakar S, Shea KJ (1995) Alkylene-bridged polysilsesquioxane aerogels: highly porous hybrid organic-inorganic materials. J Non-Cryst Solids 186:44–53
Li Z, Tolbert S, Loy DA (2013) Hybrid organic-inorganic membranes on porous supports by size exclusion and thermal sintering of fluorescent polyphenylsilsesquioxane nanoparticles. Macromol Mater Eng 298(7):715–721. https://doi.org/10.1002/mame.201200091
Deng T-S, Marlow F (2012) Synthesis of monodisperse polystyrene@vinyl-SiO2 core–shell particles and hollow SiO2 spheres. Chem Mater 24(3):536–542. https://doi.org/10.1021/cm203099m
Acknowledgements
We thank Dr. Michael Zimmer for the CP-MAS-NMR measurements and Susanne Harling for elemental analyses.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Highlights
-
The paper shows for the first time the continuous, wet chemical preparation of ORMOSIL micro-and nanoparticles applying a microjet reactor.
-
No additives for the stabilization of the particles are required.
-
The particles can be prepared with yields up to 23 g min−1.
-
Well-defined particle morphologies were obtained of methyl-, ethyl-, propyl-, vinyl-, and phenyl-ORMOSILs as well as pure silica particles.
-
The condensation degrees of the particles vary depending on the organic substitution pattern at the silicon atom.
Electronic supplementary material
Rights and permissions
About this article
Cite this article
Odenwald, C., Kickelbick, G. Additive-free continuous synthesis of silica and ORMOSIL micro- and nanoparticles applying a microjet reactor. J Sol-Gel Sci Technol 89, 343–353 (2019). https://doi.org/10.1007/s10971-018-4626-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10971-018-4626-x