Abstract
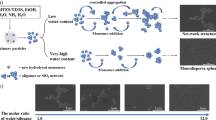
A modified preparation of silica nanoparticles via sol–gel process was described. The ability to control the particle size and distribution was found highly dependent on mixing modes of the reactants and drying techniques. The mixture of tetraethoxysilane and ethanol followed by addition of water (Mode-A) produced monodispersed powder with an average particle size of 10.6 ± 1.40 nm with a narrow size distribution. The freeze drying technique (FD) further improved the quality of powder. In addition, the freeze dried samples have shown unique TGA decomposition steps which might be related to the well-defined structure of silica nanoparticles as compared to the heat dried samples. DSC analysis showed that FD preserved the silica surface with low shrinkage and generated remarkably well-order, narrow and bigger pore size and pore volume and also large endothermic enthalpies (ΔH FD = −688 J g−1 vs. ΔH HD = −617 J g−1) that lead to easy escape of physically adsorbed water from the pore at lower temperature.







Similar content being viewed by others
References
Iller RK (1979) The chemistry of silica. Wiley, New York
Hench LL, West JK (1990) Chem Rev 90:33
Matsoukas T, Gulari E (1998) J Colloid Interface Sci 124:252. doi:10.1016/0021-9797(88)90346-3
Matsoukas T, Gulari E (1989) J Colloid Interface Sci 132:13. doi:10.1016/0021-9797(89)90210-5
Bogush GH, Zukoski CF (1991) J Colloid Interface Sci 142:19. doi:10.1016/0021-9797(91)90030-C
Chu L, Tejedor-Tejedor MI, Anderson MA (1997) Microporous Mater 8:207. doi:10.1016/S0927-6513(96)00068-5
Meixner DL, Dyer PN (1999) J Sol-Gel Sci Technol 14:223. doi:10.1023/A:1008774827602
Colomer MT, Anderson MA (2001) J Non-Cryst Solids 290:93. doi:10.1016/S0022-3093(01)00815-8
Enomoto N, Kumagai A, Hojo J (2005) J Ceram Soc Jpn 113:340. doi:10.2109/jcersj.113.340
Stöber W, Fink A, Bohn E (1968) J Colloid Interface Sci 26:62. doi:10.1016/0021-9797(68)90272-5
Van Helden AK, Jansen JW, Vrij A (1981) J Colloid Interface Sci 81:354. doi:10.1016/0021-9797(81)90417-3
Tan CG, Bowen BD, Epstein N (1987) J Colloid Interface Sci 118:290. doi:10.1016/0021-9797(87)90458-9
Enomoto N, Koyano T, Nakagawa Z (1996) Ultrason Sonochem 3:S105. doi:10.1016/1350-1477(96)00004-W
Chen SL, Dong P, Yang GH (1997) J Colloid Interface Sci 189:268. doi:10.1006/jcis.1997.4809
Sadasivan S, Rasmussen DH, Chen FP, Kannabiran RK (1998) Colloid Surf A 132:45. doi:10.1016/S0927-7757(97)00148-9
Lee K, Sathyagal AN, McCormick AV (1998) Colloid Surf A 144:115. doi:10.1016/S0927-7757(98)00566-4
Park SK, Kim KD, Kim HT (2002) Colloid Surf A 197:7. doi:10.1016/S0927-7757(01)00683-5
Green DL, Jayasundara S, Lam YF, Harris MT (2003) J Non-Cryst Solids 315:166. doi:10.1016/S0022-3093(02)01577-6
Green DL, Lin JS, Lam YF, Hu MZC, Schaefer DW, Harris MT (2003) J Colloid Interface Sci 266:346. doi:10.1016/S0021-9797(03)00610-6
Nagao D, Osuzu H, Yamada A, Mine E, Kobayashi Y, Konno M (2004) J Colloid Interface Sci 279:143. doi:10.1016/j.jcis.2004.06.041
Kim SS, Kim HS, Kim SG, Kim WS (2004) Ceram Int 30:171. doi:10.1016/S0272-8842(03)00085-3
Rahman IA, Vejayakumaran P, Sipaut CS, Ismail J, Abu Bakar M, Adnan R, Chee CK (2006) Ceram Int 32:691. doi:10.1016/j.ceramint.2005.05.004
Rahman IA, Vejayakumaran P, Sipaut CS, Ismail J, Abu Bakar M, Adnan R, Chee CK (2007) Colloid Surf A 294:102. doi:10.1016/j.colsurfa.2006.08.001
Bogush GH, Zukoski CF (1991) J Colloid Interface Sci 142:1. doi:10.1016/0021-9797(91)90029-8
Greenwood NN, Earnshaw A (1998) Chemistry of the elements, 2nd edn. Butterworth Heinemann, Oxford
Gregory JK, Clary DC, Liu K, Brown MG, Saykally RJ (1997) Science 275:814. doi:10.1126/science.275.5301.814
Brinker CJ, Scherer GW (1990) Sol-gel science. Academic Press, San Diego
Chen G, Wang W (2007) Dry Technol 25:29. doi:10.1080/07373930601161179
Choi MJ, Briançon S, Andrieu J, Min SG, Fessi H (2004) Dry Technol 22:335. doi:10.1081/DRT-120028238
Brunauer S, Deming LS, Deming WE, Teller E (1940) J Am Chem Soc 62:1723. doi:10.1021/ja01864a025
Vansant EF, Van Der Voort P, Vrancken KC (1995) Characterization and chemical modification of the silica surface. Elsevier, Amsterdam
Barrett EP, Joyner LG, Halenda PP (1951) J Am Chem Soc 73:373. doi:10.1021/ja01145a126
Yuaga S, Okabayahi M, Ohno H, Suzuki K, Kusumoto K (1988) US Patent 4, 764:497
Ek S, Root A, Peussa M, Niinistö L (2001) Thermochim Acta 379:201. doi:10.1016/S0040-6031(01)00618-9
Acknowledgment
The authors appreciate Ministry of Higher Education for financial support of this research under Fundamental Research Grant Scheme (FRGS, Grant No: 203/PKIMIA/671174). M. Jafarzadeh would like to express his gratitude to Universiti Sains Malaysia (USM) for USM-Fellowship.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Jafarzadeh, M., Rahman, I.A. & Sipaut, C.S. Synthesis of silica nanoparticles by modified sol–gel process: the effect of mixing modes of the reactants and drying techniques. J Sol-Gel Sci Technol 50, 328–336 (2009). https://doi.org/10.1007/s10971-009-1958-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10971-009-1958-6