Abstract
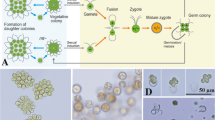
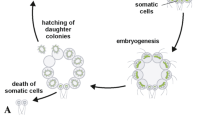
Cryopreservation is needed to ensure long-term storage without any genetic drift and contamination. To conserve Ulva species, gametophytic thalli of Ulva prolifera (Chlorophyta) were first cryopreserved at −196 °C using the two-step cooling method with cooling rate of 1 °C min−1 to −40 °C. Three cryoprotectants (dimethyl sulfoxide (DMSO), glycerol, and proline) at five concentrations (5, 10, 15, 20, and 25 %) and mixed cryoprotectants were assessed to determine the optimal solution. The mixture of cryoprotectants with the highest viability was 10 % glycerol in combination with 5 % DMSO and 5 % proline, with a viability of 89.2 %. The cryoprotectant treated singly with the highest viability was 20 % glycerol with a viability of 91.6 %. However, there was no statistically significant difference between 20 % glycerol and the mixed treatment. An evaluation of long-term storage in liquid nitrogen showed very high viability of 91.2–92.1 % with no statistically significant effect from storage time up to 120 days. The specimens developed normally in culture after cryopreservation for 120 days with the rate of gametogenesis reaching 95.7 % on the fourth day of culture. The released gametes developed normally into gametothalli. In conclusion, the use of glycerol as a cryoprotectant was very effective for cryopreservation of the gametothalli of U. prolifera. With 20 % glycerol, viability was very high as 91.6 %. The results suggest that gametothalli of U. prolifera can be cryopreserved completely by a treatment with 15–25 % glycerol singly as well as by a mixture of cryoprotectants with usual efficiency as a cryoprotectant.




Similar content being viewed by others
References
Cañavate JP, Lubian LM (1995) Relationship between cooling rates, cryoprotectant concentrations and salinities in the cryopreservation of marine microalgae. Mar Biol 124:325–334
Cho JY, Kwon EH, Choi JS, Hong SY, Shin HW, Hong YK (2001) Antifouling activity of seaweed extracts on the green alga Enteromorpha prolifera and the mussel Mytilus edulis. J Appl Phycol 13:117–125
Choi YH, Nam TJ, Kuwano K (2013) Cryopreservation of gametophytic thalli of Porphyra yezoensis (Rhodophyceae) by one-step fast cooling. J Appl Phycol 25:531–535
Dan A, Hiraoka M, Ohno M, Critchley AT (2002) Observations on the effect of salinity and photon fluence rate on the induction of sporulation and rhizoid formation in the green alga Enteromorpha prolifera (Müller) J. Agardh (Chlorophyta, Ulvales). Fish Sci 68:1182–1188
Fenwick C, Day JG (1992) Cryopreservation of Tetraselmis suecica cultured under different nutrients regimes. J Appl Phycol 4:105–109
Helliot B, Mortain-Bertrand A (1999) Accumulation of proline in Dunaliella salina (Chlorophyceae) in response to light transition and cold adaptation. Effect on cryopreservation. Cryo-Lett 20:287–296
Hiraoka M, Oka N (2008) Tank cultivation of Ulva prolifera in deep seawater using a new “germling cluster” method. J Appl Phycol 20:97–102
Hubálek Z (2003) Protectants used in the cryopreservation of microorganisms. Cryobiology 46:205–229
Kono S, Kuwano K, Ninomiya M, Onishi J, Saga N (1997) Cryopreservation of Enteromorpha intestinalis (Ulvales, Chlorophyta) in liquid nitrogen. Phycologia 36:76–78
Kono S, Kuwano K, Saga N (1998) Cryopreservation of Eisenia bicyclis (Laminariales, Phaeophyta) in liquid nitrogen. J Mar Biotech 6:220–223
Kuwano K, Saga N (2000) Cryopreservation of marine algae: Applications in Biotechnology. In: Fingerman M, Nagabhushanam R (eds) Recent advances in marine biotechnology, vol 4, Aquaculture. Science, New Hamsphire, pp 23–40
Kuwano K, Aruga Y, Saga N (1993) Cryopreservation of the conchocelis of the marine alga Porphyra yezoensis Ueda (Rhodophyta) in liquid nitrogen. Plant Sci 94:215–225
Kuwano K, Aruga Y, Saga N (1994) Cryopreservation of the conchocelis of Porphyra (Rhodophyta) by applying a simple prefreezing system. J Phycol 30:556–570
Kuwano K, Aruga Y, Saga N (1996) Cryopreservation of clonal gametophytic thalli of Porphyra (Rhodophyta). Plant Sci 116:117–124
Kuwano K, Kono S, Jo YH, Shin JA, Saga N (2004) Cryopreservation of the gametophytic cells of Laminariales (Phaeophyta) in liquid nitrogen. J Phycol 40:606–610
Lin A, Shen S, Wang J, Yan B (2008) Reproduction diversity of Enteromorpha prolifera. J Integr Plant Biol 50:622–629
Mantri VA, Singh RP, Bijo A, Kumari P, Reddy C, Jha B (2011) Differential response of varying salinity and temperature on zoospore induction, regeneration and daily growth rate in Ulva fasciata (Chlorophyta, Ulvales). J Appl Phycol 23:243–250
Mazur P (1984) Freezing of living cells: mechanisms and implications. Am J Physiol 247:C125–C142
Mortain-Bertrand A, Etchart F, Boucaud MTD (1996) A method for the cryoconservation of Dunaliella salina (Chlorophyceae): effect of glycerol and cold adaptation. J Phycol 32:346–352
Nakanishi K, Deuchi K, Kuwano K (2012) Cryopreservation of four valuable strains of microalgae, including viability and characteristics during 15 years of cryostorage. J Appl Phycol 24:1381–1385
Poncet JM, Véron B (2003) Cryopreservation of the unicellular marine alga, Nannochloropsis oculata. Biotech Lett 25:2017–2022
Provasoli L (1968) Media and prospects for the cultivation of marine algae. In: Watanabe A, Hattori A (eds) Cultures and collections of algae. Jpn Soc Plant Physiol, Hakone, Japan, pp 63–75
Saga N, Sakanishi Y, Ogishima T (1989) Method for quick evaluation of cell viability in marine macroalgae. Jpn J Phycol 37:129–136
Taylor R, Fletcher RL (1998) Cryopreservation of eukaryotic algae—a review of methodologies. J Appl Phycol 10:481–501
Taylor R, Fletcher RL (1999) A simple method for the freeze-preservation of zoospores of the green macroalga Enteromorpha intestinalis. J Appl Phycol 11:257–262
Van den Hoek C, Mann DG, Jahns HM (1995) Algae: an introduction to phycology. Cambridge University Press, Cambridge
Van der Meer JP, Simpson FJ (1984) Cryopreservation of Gracilaria tikvahiae (Rhodophyta) and other macrophytic marine algae. Phycologia 23:195–202
Wichard T, Oertel W (2010) Gametogenesis and gamete release of Ulva mutabilis and Ulva lactuca (Chlorophyta): regulatory effects and chemical characterization of the “swarming inhibitor”. J Phycol 46:248–259
Zhang QS, Cong YZ, Qu SC, Luo SJ, Li XJ, Tang XX (2007) A simple and highly efficient method for the cryopreservation of Laminaria japonica (Phaeophyceae) germplasm. Eur J Phycol 42:209–213
Zhang XW, Mao YZ, Zhuang ZM, Liu SF, Wang QY, Ye NH (2008) Morphological characteristics and molecular phylogenetic analysis of green tide Enteromorpha sp. occurred in the Yellow Sea. J Fish Sci China 15:822–829
Zhang X, Wang H, Mao Y, Liang C, Zhuang Z, Wang Q, Ye N (2010) Somatic cells serve as a potential propagule bank of Enteromorpha prolifera forming a green tide in the Yellow Sea, China. J Appl Phycol 22:173–180
Zhang J, Huo Y, Yu K, Chen Q, He Q, Han W, Chen L, Cao J, Shi D, He P (2013) Growth characteristics and reproductive capability of green tide algae in Rudong coast, China. J Appl Phycol 25:795–803
Zhuang Y, Guo J, Chen L, Li D, Liu J, Ye N (2012) Microwave-assisted direct liquefaction of Ulva prolifera for bio-oil production by acid catalysis. Bioresour Technol 116:113–139
Zhuang Y, Gong X, Zhang W, Gao W (2015) Cryopreservation of filaments of Scytosiphon lomentaria by vitrification. J Appl Phycol 27:1337–1342
Acknowledgments
This work was supported by a grant from Marine Biotechnology Program funded by Ministry of Oceans and Fisheries of Korean Government.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Lee, Y.N., Nam, K.W. Cryopreservation of gametophytic thalli of Ulva prolifera (Ulvales, Chlorophyta) from Korea. J Appl Phycol 28, 1207–1213 (2016). https://doi.org/10.1007/s10811-015-0620-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10811-015-0620-7