Abstract
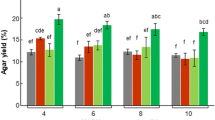
This study explores the possibility of producing ethanol using the acid hydrolysate of three abundant agar-containing red seaweeds (agarophytes): Gelidium amansii, Gracilaria tenuistipitata, and Gracilariopsis chorda. The main component in the seaweed samples was agar, which ranged from 20 to 51 % (g g−1 dry weight). After optimizing acid hydrolysis, 100 g of seaweed was hydrolyzed at 130 °C for 15 min with 0.2 M H2SO4. Then, 120 mL of a 1:2 mixture of the hydrolysate broth and basal medium was fermented in a 200-mL bottle at 30 °C for 96 h. Of the three seaweeds, G. amansii had the best ethanol yield, producing 0.23 g g−1 of galactose or 45 % of the theoretical yield. This yield increased to 60 % after detoxification of the hydrolysate with activated carbon.


Similar content being viewed by others
References
Adams MJ, Gallagher JA, Donnison IS (2009) Fermentation study on Saccharina latissima for bioethanol production considering variable pre-treatments. J Appl Phycol 21:569–574
Afionis S, Stringer LC (2012) European Union leadership in biofuels regulation: Europe as a normative power? J Clean Prod 32:114–123
Alves LA, Felipe MGA, Silva JB, Silva SS, Prata AMR (1998) Pre treatment of sugar cane bagasse hemicellulose hydrolysate for xylitol production by Candida guilliermondii. J Appl Biochem Biotechnol 70–72:89–98
Boucher P (2012) The role of controversy, regulation and engineering in UK biofuel development. Energy Pol 42:148–154
Bruhn A, Dahl J, Nielsen HB, Nikolaisen L, Rasmussen MB, Markager S, Olesen B, Arias C, Jensen PD (2011) Bioenergy potential of Ulva lactuca: biomass yield, methane production and combustion. Bio Tech 102:2595–2604
Chaplin MF (1986) Monosaccharide. In: Chaplin MF, Kennedy JF (eds) Carbohydrate analysis: a practical approach. IRC, Oxford, pp 1–36
de Ruiter GA, Rudolph B (1997) Carrageenan biotechnology. Trends Food Sci Technol 8:389–395
Deverell R, McDonnell K, Ward S, Devlin G (2009) An economic assessment of potential ethanol production pathways in Ireland. Energy Pol 37:3993–4002
de Vrije T, Bakker RR, Budde AW, Lai MH, Mars AE, Claassen PA (2009) Efficient hydrogen production from lignocellulosic energy crop Miscanthus by extreme thermophilic bacteria Caldicellulosiruptor saccharolyticus and Thermotoga neapolitana. Biotechnol Biofuel 2:12. doi:10.1186/1754-6834-2-12
Dixon RK, McGowan E, Onysko G, Scheer RM (2010) US energy conservation and efficiency policies: challenges and opportunities. Energy Pol 38:6398–6408
Gray KA, Zhao L, Emptage M (2006) Bioethanol. Opin Chem Biol 10:141–146
Horn SJ, Aasen IM, Ostgaard K (2008) Ethanol production from seaweed extract. J Ind Microbiol Biotechnol 25:249–254
Jol CN, Neiss TG, Penninkhof B, Rudolph B, Ruiter GAD (1999) A novel high-performance anion-exchange chromatographic method for the analysis of carrageenans and agars containing 3,6-anhydrogalactose. Anal Biochem 268:213–222
Klinke HB, Thomsen AB, Ahring BK (2004) Inhibition of ethanol-producing yeast and bacteria by degradation products produced during pre-treatment of biomass. Appl Microbiol Biotechnol 66:10–26
Kochert G (1978) Carbohydrate determination by the phenol-sulfuric acid method. In: Hellebust JA, Craigie JS (eds) Handbook of phycological methods, vol II, Physiological and biochemical methods. Cambridge University Press, Cambridge, pp 95–97
Larsson S, Palmqvist E, Hagerdal BH, Tengborg C, Stenberg K, Zacchi G, Nilvebrant (1999) The generation of fermentation inhibitors during dilute acid hydrolysis of softwood. Enzyme Microb Technol 24:151–159
Levring T, Hoppe HA, Schmid OJ (1969) Marine algae. A survey of research and utilization. Botanica marina handbook, vol. 1. Cram, De Gruyter & Co, , Hamburg, p 421
McHugh DJ (1987) Production and utilization of products from commercial seaweeds. Fisheries Technical Paper, FAO, Rome
McHugh D (2003) A guide to the seaweed industry. Fisheries Technical Paper, FAO, Rome
Meinita DNM, Kang JY, Jeong GT, Koo HM, Park SM, Hong YK (2012a) Bioethanol production from the acid hydrolysate of the carrageenophyte Kappaphycus alvarezii (cottonii). J Appl Phycol 24:857–862
Meinita DNM, Jeong GT, Hong YK (2011) Comparison of sulfuric and hydrochloric acids as catalysts in hydrolysis of Kappaphycus alvarezii (cottonii). Bioproc Biosyst Eng 35:123–128
Meinita DNM, Jeong GT, Hong YK (2012b) Detoxification of acidic catalyzed hydrolysate of Kappaphycus alvarezii (cottonii). Bioproc Biosyst Eng 3:93–98
Mouradi-Givernaud A, Amina-Hassani L, Givernaud T, Lemoine Y, Benharbet O (1999) Biology and agar composition of Gelidium sesquipedale harvested along the Atlantic coast of Morocco. Hydrobiologia 5:391–395
Orosco CA, Anong C, Nukaya M, Ohno M, Sawamura M, Kusunose H (1992) Yield and physical characteristics of Agar from Gracilaria chorda Holmes: comparison with those from Southeast Asian species. Nippon Suisan Gakkaishi 58:1711–1716
Percival E (1979) The polysaccharide of green, red and brown seaweeds: their basic structure, biosynthesis and function. Br Phycol J 14:103–117
Prescott SC, Dun CG (1959) Industrial microbiology, 3rd edn. McGraw-Hill, New York, p 120
Wi SG, Kim HJ, Mahadevan SA, Yang DJ, Bae HJ (2009) The potential value of the seaweed Ceylon moss (Gelidium amansii) as an alternative bioenergy resource. Bioresour Technol 100:6658–6660
Yokoya NS, West JA, Luchi AE (2004) Effects of plant growth regulators on callus formation, growth and regeneration in axenic tissue cultures of Gracilaria tenuistipitata and Gracilaria perplexa (Gracilariales, Rhodophyta). Phycol Res 52:244–254
Acknowledgments
This research was supported by a grant from the Samsung Advanced Institute of Technology, Korea. We are grateful for a postdoctoral fellowship from the Pukyong National University (MNAK) and the Brain Busan 21 program for graduate support. We thank to the Jenderal Soedirman University (UNSOED), Indonesia, for the collaboration research (Riset Unggulan UNSOED).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Meinita, M.D.N., Marhaeni, B., Winanto, T. et al. Comparison of agarophytes (Gelidium, Gracilaria, and Gracilariopsis) as potential resources for bioethanol production. J Appl Phycol 25, 1957–1961 (2013). https://doi.org/10.1007/s10811-013-0041-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10811-013-0041-4