Abstract
Introduction
Alterations in mucosal histamine degradation play an important role in various gastrotinestinal diseases including colonic adenoma. In humans, histamine can be catabolized either by oxidative deamination by diamine oxidase (DAO) or by ring methylation by histamine N-methyltransferase (HNMT). The significance of HNMT in this context was investigated for the first time in this project.
Methods
About 94 colonic biopsies were endoscopically obtained from 23 patients suffering from colonic adenoma and 26 biopsies from six healthy individuals. Each sample was mechanically homogenized, homogenates were cleared by centrifugation and used for determination of protein and histamine concentrations and enzyme activities of DAO and HNMT by radiometric assay.
Results
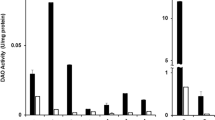
In adenoma patients DAO activities were slightly and HNMT activities were significantly decreased in normal mucosa compared to controls. Activities of both enzymes were significantly lower in adenoma tissue than in healthy mucosa in the same patients. A significant correlation was found between HNMT and DAO in all investigated samples. Histamine concentrations were elevated in adenoma patients.
Conclusions
Histamine catabolism is decreased in the colonic mucosa of patients with colonic adenoma.




Similar content being viewed by others
References
Markowitz SD, Dawson DM, Willis J, Willson JK (2002) Focus on colon cancer. Cancer Cell 1:233–236
Burt RW (2000) Colon cancer screening. Gastroenterology 119:837–253
Seitz HK, Simanowski UA, Homann N, Waldherr R (1998) Cell proliferation and its evaluation in the colorectal mucosa: effect of ethanol. Z Gastroenterol 36:645–655
Prichard PJ, Tjandra JJ (1998) Colorectal cancer. Med J Aust 169:493–498
Hardy RG, Meltzer SJ, Jankowski JA (2000) ABC of colorectal cancer. Molecular basis for risk factors. BMJ 321:886–889
Leslie A, Carey FA, Pratt NR, Steele RJC (2002) The colorectal adenoma-carcinoma sequence. Br J Surg 89:845–860
Villavicencio RT, Rex DK (2000) Colonic adenomas: prevalence and incidence rates, growth rates, and miss rates at colonoscopy. Semin Gatrointest Dis 11:185–193
Srivastava S, Verma M, Henson DE (2001) Biomarkers for early detection of colon cancer. Clin Cancer Res 7:1118–1126
Kusche J, Mennigen R, Leisten L (1989) Early alterations of rat intestinal diamine oxidase activity by azoxymethane, an intestinal carcinogen. Agents Actions 27:218–220
Raithel M, Ulrich P, Hochberger J, Hahn EG (1998) Measurement of gut diamine oxidase activity: diamine oxidase as a new biologic marker of colorectal proliferation? Ann NY Acad Sci 859:262–266
Pearce FL (1991) Biological effects of histamine: an overview. Agents Actions 33:4–7
Norrby K (1985) Evidence of mast-cell histamine being mitogenic in intact tissue. Agents Actions 16:287–290
Argento-Cerú MP, Autouri F (1985) Localization of diamine oxidase in animal tissues. In: Mondovi B (ed) Structure and functions of amine oxidases. CRC Press, Boca Raton pp 89–104
Kusche J, Mennigen R, Erpenbach K (1988) The intestinal diamine oxidase activity under the influence of adaptive proliferation of the intestinal mucosa – a proliferation terminating principle? Agents Actions 23:354–356
Code CF (1985) Histamine – whither now? Can J Physiol Pharmacol 63:746–750
Kusche J, Mennigen R, Leisten L, Krakamp B (1988) Large bowel tumour promotion by diamine oxidase inhibition: animal model and clinical aspects. Adv Exp Med Biol 250:745–752
Pacifici GM, Donatelli P, Giuliani L (1992) Histamine N-methyltransferase: inhibition by drugs. Br J Clin Pharmac 34:322–327
Sattler J, Hesterberg R, Lorenz W, Schmidt U, Crombach M (1985) Inhibition of human and canine diamine oxidase by drugs used in an intensive care unit: relevance for clinical side effects? Agents Actions 16:91–94
Smith PK, Krohn RI, Hermanson GT, Mallia AK, Gartner FH, Provenzano MD, Fujimoto EK, Goeke NM, Olson BJ, Klenk DC (1985) Measurement of protein using bicinchoninic acid. Anal Biochem 150(1):15076–15085
Kusche J, Richter H, Hesterberg R, Schmidt J, Lorenz W (1973) Comparison of the 14C-Putrescine assay with the NADH test for the determination of diamine oxidase: description of a standard procedure with a high precision and an improved accuracy. Agents Actions 3:148–156
Schwelberger HG, Klocker J, Sattler J, Bodner E (1995) Determination of the activity of diamine oxidase in extremely small tissue samples. Inflamm Res 44:94–95
Brown DD, Tomchick R, Axelrod J (1959) The distribution and properties of a histamine-methylating enzyme. J Biol Chem 234:2948–2950
Barth H, Lorenz W, Niemeyer I (1973) Inhibition and activation of histamine methyltransferase by methylated histamines. Hoppe Seylers Z Physiol Chem 354:1021–1026
Beaven MA, Robinson-White A, Roderick NB, Kauffmann GL (1982) The demonstration of histamine release in clinical conditions: a review of past and present assay procedures. Klin Wochenschr 60:873–881
Verburg KM, Bowsher RR, Henry DP (1983) A new radioenzymatic assay for histamine using purified histamine N-methyltransferase. Life Sci 32:2855–2867
Kufner MA, Ulrich P, Raithel M, Schwelberger HG (2001) Determination of the histamine degradation capacity in extremely small human colon samples. Inflamm Res 50(Suppl 2):96–97
Hesterberg R, Sattler J, Lorenz W, Stahlknecht CD, Barth H, Crombach M (1984) Histamine content, diamine oxidase activity and histamine methyltransferase activity in human tissues: fact or fictions? Agents Actions 14:325–334
Schwelberger HG, Bodner E (1997) Purification and characterization of diamine oxidase from porcine kidney and intestine. Biochim Biophys Acta 1340:152–164
Brown DD, Tomchick R, Axelrod J (1959) The distribution and properties of a histamine-methylating enzyme. J Biol Chem 234:2948–2950
Raithel M, Horauf AM, Matek M, Baenkler HW (1989) Kinetics of histamine released from rectal mucosa. Agents Actions 28:164–167
Backhaus B, Weidenhiller M, Bijlsma P, Hahn EG, Raithel M (2004) Evaluation of spontaneous histamine release from colorectal mucosa in patients with colorectal adenoma, patients with gastrointestinally mediated allergy and in a healthy control group. Inflamm Res 53(Suppl 1):87–88
Petersen J, Raithel M, Schwelberger HG (2002) Histamine N-methyltransferase and diamine oxidase gene polymorphisms in patients with inflammatory and neoplasitc intstinal dieases. Inflamm Res 51(Suppl 1):91–92
Adams WJ, Lawson JA, Morris DL (1994) Cimetidine inhibits in vivo growth of human colon cancer and reverses histamine stimulated in vitro and in vivo growth. Gut 35:1632–1636
Watson SA, Wilkinson LJ, Robertson JFR, Hardcastle JD (1993) Effect of histamine on the growth of human gastrointestinal tumour: reversal by cimetidine. Gut 34:1091–1096
Bodmer S, Imark C, Kneubühl M (1999) Biogenic amines in food: histamine and food processing. Inflamm res 48:296–300
Wantke F, Jarisch J, Götz M (1993) Histamine-free diet: treatment of choice for histamine-induced food intolerance and supporting treatment for chronic headaches. Clin Exp Allergy 23:982–985
Raithel M, Kufner M, Ulrich P, Hahn EG (1999) The involvement of the histamine degradation pathway by diamine oxidase in manifest gastrointestinal allergies. Inflamm Res 48(Suppl 1):75–76
Weidenhiller M, Raithel M, Winterkamp S, Otte P, Stolper J, Hahn EG (2000) Methylhistamine in Crohn´s disease (CD): increased production and elevated urine excretion correlates with disease activity. Inflamm Res 49(Suppl 1):35–36
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kuefner, M.A., Schwelberger, H.G., Hahn, E.G. et al. Decreased Histamine Catabolism in the Colonic Mucosa of Patients with Colonic Adenoma. Dig Dis Sci 53, 436–442 (2008). https://doi.org/10.1007/s10620-007-9861-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10620-007-9861-x