Abstract
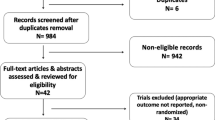
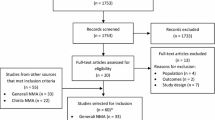
The goal of this study was to compare the efficacy and toxicity of chemotherapy to exemestane plus everolimus (EXE/EVE) through a network meta-analysis (NMA) of randomized controlled trials. NMA methods extend standard pairwise meta-analysis to allow simultaneous comparison of multiple treatments while maintaining randomization of individual studies. The method enables “direct” evidence (i.e., evidence from studies directly comparing two interventions) and “indirect” evidence (i.e., evidence from studies that do not compare the two interventions directly) to be pooled under the assumption of evidence consistency. We used NMA to evaluate progression-free survival (PFS) and time to progression (TTP) curves in 34 studies, and response rate (RR) and the hazard ratios (HRs) of the PFS/TTP in 36 studies. A number needed to treat (NNT) analysis was also performed as well as descriptive comparison of reported toxicities. The NMA for PFS/TTP curves and for HR shows EXE/EVE is more efficacious than capecitabine plus sunitinib, CMF, megestrol acetate and tamoxifen, with an average of related-PFS/TTP difference ranging from about 10 months for capecitabine plus sunitinib to more than 6 months for tamoxifen. The NMA for overall RR shows that EXE/EVE provides a better RR than bevacizumab plus capecitabine, capecitabine, capecitabine plus sorafenib, capecitabine plus sunitinib, CMF, gemcitabine plus epirubicin plus paclitaxel, EVE plus tamoxifen, EXE, FEC, megestrol acetate, mitoxantrone, and tamoxifen. Finally, the NMA for NNT shows that EXE/EVE is more beneficial as compared to BMF, capecitabine, capecitabine plus sunitinib, CMF, FEC, megestrol acetate, mitoxantrone, and tamoxifen. The combination of EXE/EVE as first- or second-line therapy for ER+ve/HER2−ve metastatic breast cancer is more efficacious than several chemotherapy regimens that were reported in the literature. Toxicities also favored EXE/EVE in most instances.





Similar content being viewed by others
References
Roche H, Vahdat LT (2011) Treatment of metastatic breast cancer: second line and beyond. Ann Oncol 22:1000–1010
Fernandez Y, Cueva J, Palomo AG et al (2010) Novel therapeutic approaches to the treatment of metastatic breast cancer. Cancer Treat Rev 36:33–42
Baselga J, Campone M, Piccart M et al (2012) Everolimus in postmenopausal hormone-receptor-positive advanced breast cancer. N Engl J Med 366:520–529
Yardley DA, Noguchi S, Pritchard KI et al (2013) Everolimus plus exemestane in postmenopausal patients with HR(+) breast cancer: BOLERO-2 final progression-free survival analysis. Adv Ther 30:870–884
Burris HA III, Lebrun F, Rugo HS et al (2013) Health-related quality of life of patients with advanced breast cancer treated with everolimus plus exemestane versus placebo plus exemestane in the phase 3, randomized, controlled, BOLERO-2 trial. Cancer 119:1908–1915
Campone M, Bachelot T, Gnant M et al (2013) Effect of visceral metastases on the efficacy and safety of everolimus in postmenopausal women with advanced breast cancer: subgroup analysis from the BOLERO-2 study. Eur J Cancer 49:2621–2632
Gradishar WJ, Anderson BO, Blair SL et al (2014) Breast cancer version 3.2014. J Natl Compr Cancer Netw 12:542–590
Cardoso F, Harbeck N, Fallowfield L et al (2012) Locally recurrent or metastatic breast cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol 23(Suppl 7):vii11–vii19
Lin NU, Thomssen C, Cardoso F et al (2013) International guidelines for management of metastatic breast cancer (MBC) from the European School of Oncology (ESO)-MBC Task Force: surveillance, staging, and evaluation of patients with early-stage and metastatic breast cancer. Breast 22:203–210
Bachelot T, McCool R, Duffy S et al (2014) Comparative efficacy of everolimus plus exemestane versus fulvestrant for hormone-receptor-positive advanced breast cancer following progression/recurrence after endocrine therapy: a network meta-analysis. Breast Cancer Res Treat 143:125–133
Partridge AH, Rumble RB, Carey LA et al (2014) Chemotherapy and targeted therapy for women with human epidermal growth factor receptor 2-negative (or unknown) advanced breast cancer: American Society of Clinical Oncology Clinical Practice Guideline. J Clin Oncol 32:3307–3329
Hoaglin DC, Hawkins N, Jansen JP et al (2011) Conducting indirect-treatment-comparison and network-meta-analysis studies: report of the ISPOR Task Force on Indirect Treatment Comparisons Good Research Practices: part 2. Value Health 14:429–437
Jansen JP, Fleurence R, Devine B et al (2011) Interpreting indirect treatment comparisons and network meta-analysis for health-care decision making: report of the ISPOR Task Force on Indirect Treatment Comparisons Good Research Practices: part 1. Value Health 14:417–428
Thorlund K, Zafari Z, Druyts E et al (2014) The impact of incorporating Bayesian network meta-analysis in cost-effectiveness analysis—a case study of pharmacotherapies for moderate to severe COPD. Cost Eff Resour Alloc 12:8
Zafari Z, Thorlund K, FitzGerald JM et al (2014) Network vs. pairwise meta-analyses: a case study of the impact of an evidence-synthesis paradigm on value of information outcomes. Pharmacoeconomics 32:995–1004
Dixon AR, Jackson L, Chan S et al (1992) A randomised trial of second-line hormone vs single agent chemotherapy in tamoxifen resistant advanced breast cancer. Br J Cancer 66:402–404
De Laurentiis M, Cancello G, D’Agostino D et al (2008) Taxane-based combinations as adjuvant chemotherapy of early breast cancer: a meta-analysis of randomized trials. J Clin Oncol 26:44–53
Baselga J, Segalla JG, Roche H et al (2012) Sorafenib in combination with capecitabine: an oral regimen for patients with HER2-negative locally advanced or metastatic breast cancer. J Clin Oncol 30:1484–1491
Cardoso F, Costa A, Norton L et al (2014) ESO-ESMO 2nd international consensus guidelines for advanced breast cancer (ABC2). Breast 23:489–502
Andre F, Neven P, Marinsek N et al (2014) Disease management patterns for postmenopausal women in Europe with hormone-receptor-positive, human epidermal growth factor receptor-2 negative advanced breast cancer. Curr Med Res Opin 30:1007–1016
Ingle JN (2013) Postmenopausal women with hormone receptor-positive breast cancer: balancing benefit and toxicity from aromatase inhibitors. Breast 22(Suppl 2):S180–S183
Cardoso F, Bischoff J, Brain E et al (2013) A review of the treatment of endocrine responsive metastatic breast cancer in postmenopausal women. Cancer Treat Rev 39:457–465
Cope S, Zhang J, Saletan S et al (2014) A process for assessing the feasibility of a network meta-analysis: a case study of everolimus in combination with hormonal therapy versus chemotherapy for advanced breast cancer. BMC Med 12:93
Wilkerson J, Fojo T (2009) Progression-free survival is simply a measure of a drug’s effect while administered and is not a surrogate for overall survival. Cancer J 15:379–385
Qi WX, Shen Z, Lin F et al (2013) Paclitaxel-based versus docetaxel-based regimens in metastatic breast cancer: a systematic review and meta-analysis of randomized controlled trials. Curr Med Res Opin 29:117–125
O’Shaughnessy J, Gradishar WJ, Bhar P, Iglesias J (2013) Nab-paclitaxel for first-line treatment of patients with metastatic breast cancer and poor prognostic factors: a retrospective analysis. Breast Cancer Res Treat 138:829–837
Gradishar WJ, Krasnojon D, Cheporov S et al (2012) Phase II trial of nab-paclitaxel compared with docetaxel as first-line chemotherapy in patients with metastatic breast cancer: final analysis of overall survival. Clin Breast Cancer 12:313–321
Belfiglio M, Fanizza C, Tinari N et al (2012) Meta-analysis of phase III trials of docetaxel alone or in combination with chemotherapy in metastatic breast cancer. J Cancer Res Clin Oncol 138:221–229
Xu HB, Xu Q, Li L (2011) A literature-based meta-analysis taxane-based doublet versus single-agent taxane chemotherapy in patients with advanced breast cancer. J Cancer Res Clin Oncol 137:1005–1013
Brufsky AM, Hurvitz S, Perez E et al (2011) RIBBON-2: a randomized, double-blind, placebo-controlled, phase III trial evaluating the efficacy and safety of bevacizumab in combination with chemotherapy for second-line treatment of human epidermal growth factor receptor 2-negative metastatic breast cancer. J Clin Oncol 29:4286–4293
O’Shaughnessy J, Miles D, Vukelja S et al (2002) Superior survival with capecitabine plus docetaxel combination therapy in anthracycline-pretreated patients with advanced breast cancer: phase III trial results. J Clin Oncol 20:2812–2823
Gluck S, Russell C, O’Shaughnessy J et al (2013) Treatment effect of capecitabine and docetaxel or docetaxel alone by oestrogen receptor status in patients with metastatic breast cancer: results of an exploratory analysis. Breast 22:1087–1093
Valachis A, Polyzos NP, Patsopoulos NA et al (2010) Bevacizumab in metastatic breast cancer: a meta-analysis of randomized controlled trials. Breast Cancer Res Treat 122:1–7
Cuppone F, Bria E, Vaccaro V et al (2011) Magnitude of risks and benefits of the addition of bevacizumab to chemotherapy for advanced breast cancer patients: meta-regression analysis of randomized trials. J Exp Clin Cancer Res 30:54
Gligorov J, Doval D, Bines J et al (2014) Maintenance capecitabine and bevacizumab versus bevacizumab alone after initial first-line bevacizumab and docetaxel for patients with HER2-negative metastatic breast cancer (IMELDA): a randomised, open-label, phase 3 trial. Lancet Oncol 15:1351–1360
Fleischer F, Gaschler-Markefski B, Bluhmki E (2009) A statistical model for the dependence between progression-free survival and overall survival. Stat Med 28:2669–2686
Campone M, Beck JT, Gnant M et al (2013) Health-related quality of life and disease symptoms in postmenopausal women with HR(+), HER2(−) advanced breast cancer treated with everolimus plus exemestane versus exemestane monotherapy. Curr Med Res Opin 29:1463–1473
Jerusalem G, Mariani G, Ciruelos EM, Martin M, Tjan-Heijnen VCG, Neven P, Gavila Gregori J, Michelotti A, Montemurro F, Lang I, Mardiak J, Naume B, Camozzi M, Lorizzo K, Brenski D, Conte P (2014) Everolimus in combination with exemestane in hormone receptor-positive locally advanced or metastatic breast cancer (BC) patients progressing on prior non-steroidal AI (NSAIs): BALLET study. San Antonio breast cancer symposium, P5-19-02, TX, USA, 9–13 Dec 2014
Huang H, Jiang Z, Wang T et al (2012) Single-agent capecitabine maintenance therapy after response to capecitabine-based combination chemotherapy in patients with metastatic breast cancer. Anticancer Drugs 23:718–723
Gennari A, Stockler M, Puntoni M et al (2011) Duration of chemotherapy for metastatic breast cancer: a systematic review and meta-analysis of randomized clinical trials. J Clin Oncol 29:2144–2149
Gupta S, Zhang J, Jerusalem G (2014) The association of chemotherapy versus hormonal therapy and health outcomes among patients with hormone receptor-positive, HER2-negative metastatic breast cancer: experience from the patient perspective. Expert Rev Pharmacoecon Outcomes Res 14:929–940
Ackland SP, Anton A, Breitbach GP et al (2001) Dose-intensive epirubicin-based chemotherapy is superior to an intensive intravenous cyclophosphamide, methotrexate, and fluorouracil regimen in metastatic breast cancer: a randomized multinational study. J Clin Oncol 19:943–953
Albain KS, Nag SM, Calderillo-Ruiz G et al (2008) Gemcitabine plus Paclitaxel versus Paclitaxel monotherapy in patients with metastatic breast cancer and prior anthracycline treatment. J Clin Oncol 26:3950–3957
Bachelot T, Bourgier C, Cropet C et al (2012) Randomized phase II trial of everolimus in combination with tamoxifen in patients with hormone receptor-positive, human epidermal growth factor receptor 2-negative metastatic breast cancer with prior exposure to aromatase inhibitors: a GINECO study. J Clin Oncol 30:2718–2724
Bachelot T, Bajard A, Ray-Coquard I et al (2011) Final results of ERASME-4: a randomized trial of first-line docetaxel plus either capecitabine or epirubicin for metastatic breast cancer. Oncology 80:262–268
Bergh J, Bondarenko IM, Lichinitser MR et al (2012) First-line treatment of advanced breast cancer with sunitinib in combination with docetaxel versus docetaxel alone: results of a prospective, randomized phase III study. J Clin Oncol 30:921–929
Boer K, Lang I, Llombart-Cussac A et al (2012) Vandetanib with docetaxel as second-line treatment for advanced breast cancer: a double-blind, placebo-controlled, randomized Phase II study. Investig N Drugs 30:681–687
Bonneterre J, Roche H, Monnier A et al (2002) Docetaxel vs 5-fluorouracil plus vinorelbine in metastatic breast cancer after anthracycline therapy failure. Br J Cancer 87:1210–1215
Bonneterre J, Dieras V, Tubiana-Hulin M et al (2004) Phase II multicentre randomised study of docetaxel plus epirubicin vs 5-fluorouracil plus epirubicin and cyclophosphamide in metastatic breast cancer. Br J Cancer 91:1466–1471
Brufsky A, Hoelzer K, Beck T et al (2011) A randomized phase II study of paclitaxel and bevacizumab with and without gemcitabine as first-line treatment for metastatic breast cancer. Clin Breast Cancer 11:211–220
Campone M, Dobrovolskaya N, Tjulandin S et al (2013) A three-arm randomized phase II study of oral vinorelbine plus capecitabine versus oral vinorelbine and capecitabine in sequence versus docetaxel plus capecitabine in patients with metastatic breast cancer previously treated with anthracyclines. Breast J 19:240–249
Chan S, Romieu G, Huober J et al (2009) Phase III study of gemcitabine plus docetaxel compared with capecitabine plus docetaxel for anthracycline-pretreated patients with metastatic breast cancer. J Clin Oncol 27:1753–1760
Crown JP, Dieras V, Staroslawska E et al (2013) Phase III trial of sunitinib in combination with capecitabine versus capecitabine monotherapy for the treatment of patients with pretreated metastatic breast cancer. J Clin Oncol 31:2870–2878
Del Mastro L, Venturini M, Lionetto R et al (2001) Accelerated-intensified cyclophosphamide, epirubicin, and fluorouracil (CEF) compared with standard CEF in metastatic breast cancer patients: results of a multicenter, randomized phase III study of the Italian Gruppo Oncologico Nord-Ouest-Mammella Inter Gruppo Group. J Clin Oncol 19:2213–2221
Fountzilas G, Kalofonos HP, Dafni U et al (2004) Paclitaxel and epirubicin versus paclitaxel and carboplatin as first-line chemotherapy in patients with advanced breast cancer: a phase III study conducted by the Hellenic Cooperative Oncology Group. Ann Oncol 15:1517–1526
Fountzilas G, Dafni U, Dimopoulos MA et al (2009) A randomized phase III study comparing three anthracycline-free taxane-based regimens, as first line chemotherapy, in metastatic breast cancer: a Hellenic Cooperative Oncology Group study. Breast Cancer Res Treat 115:87–99
Gradishar WJ, Kaklamani V, Sahoo TP et al (2013) A double-blind, randomised, placebo-controlled, phase 2b study evaluating sorafenib in combination with paclitaxel as a first-line therapy in patients with HER2-negative advanced breast cancer. Eur J Cancer 49:312–322
Gradishar WJ, Krasnojon D, Cheporov S et al (2009) Significantly longer progression-free survival with nab-paclitaxel compared with docetaxel as first-line therapy for metastatic breast cancer. J Clin Oncol 27:3611–3619
Hatschek T, Carlsson L, Einbeigi Z et al (2012) Individually tailored treatment with epirubicin and paclitaxel with or without capecitabine as first-line chemotherapy in metastatic breast cancer: a randomized multicenter trial. Breast Cancer Res Treat 131:939–947
Heidemann E, Stoeger H, Souchon R et al (2002) Is first-line single-agent mitoxantrone in the treatment of high-risk metastatic breast cancer patients as effective as combination chemotherapy? No difference in survival but higher quality of life were found in a multicenter randomized trial. Ann Oncol 13:1717–1729
Jones SE, Erban J, Overmoyer B et al (2005) Randomized phase III study of docetaxel compared with paclitaxel in metastatic breast cancer. J Clin Oncol 23:5542–5551
Kaufmann M, Bajetta E, Dirix LY et al (2000) Exemestane is superior to megestrol acetate after tamoxifen failure in postmenopausal women with advanced breast cancer: results of a phase III randomized double-blind trial. The Exemestane Study Group. J Clin Oncol 18:1399–1411
Langley RE, Carmichael J, Jones AL et al (2005) Phase III trial of epirubicin plus paclitaxel compared with epirubicin plus cyclophosphamide as first-line chemotherapy for metastatic breast cancer: United Kingdom National Cancer Research Institute trial AB01. J Clin Oncol 23:8322–8330
Luck HJ, Du Bois A, Loibl S et al (2013) Capecitabine plus paclitaxel versus epirubicin plus paclitaxel as first-line treatment for metastatic breast cancer: efficacy and safety results of a randomized, phase III trial by the AGO Breast Cancer Study Group. Breast Cancer Res Treat 139:779–787
Martin M, Roche H, Pinter T et al (2011) Motesanib, or open-label bevacizumab, in combination with paclitaxel, as first-line treatment for HER2-negative locally recurrent or metastatic breast cancer: a phase 2, randomised, double-blind, placebo-controlled study. Lancet Oncol 12:369–376
Miles DW, Chan A, Dirix LY et al (2010) Phase III study of bevacizumab plus docetaxel compared with placebo plus docetaxel for the first-line treatment of human epidermal growth factor receptor 2-negative metastatic breast cancer. J Clin Oncol 28:3239–3247
Miller K, Wang M, Gralow J et al (2007) Paclitaxel plus bevacizumab versus paclitaxel alone for metastatic breast cancer. N Engl J Med 357:2666–2676
von Minckwitz G, Chernozemsky I, Sirakova L et al (2005) Bendamustine prolongs progression-free survival in metastatic breast cancer (MBC): a phase III prospective, randomized, multicenter trial of bendamustine hydrochloride, methotrexate and 5-fluorouracil (BMF) versus cyclophosphamide, methotrexate and 5-fluorouracil (CMF) as first-line treatment of MBC. Anticancer Drugs 16:871–877
Norris B, Pritchard KI, James K et al (2000) Phase III comparative study of vinorelbine combined with doxorubicin versus doxorubicin alone in disseminated metastatic/recurrent breast cancer: National Cancer Institute of Canada Clinical Trials Group Study MA8. J Clin Oncol 18:2385–2394
O’Shaughnessy JA, Blum J, Moiseyenko V et al (2001) Randomized, open-label, phase II trial of oral capecitabine (Xeloda) vs. a reference arm of intravenous CMF (cyclophosphamide, methotrexate and 5-fluorouracil) as first-line therapy for advanced/metastatic breast cancer. Ann Oncol 12:1247–1254
Pajk B, Cufer T, Canney P et al (2008) Anti-tumor activity of capecitabine and vinorelbine in patients with anthracycline- and taxane-pretreated metastatic breast cancer: findings from the EORTC 10001 randomized phase II trial. Breast 17:180–185
Papadimitriou CA, Kalofonos H, Zagouri F et al (2009) Weekly docetaxel with or without gemcitabine as second-line chemotherapy in paclitaxel-pretreated patients with metastatic breast cancer: a randomized phase II study conducted by the Hellenic Co-operative Oncology Group. Oncology 77:212–216
Paridaens R, Biganzoli L, Bruning P et al (2000) Paclitaxel versus doxorubicin as first-line single-agent chemotherapy for metastatic breast cancer: a European Organization for Research and Treatment of Cancer Randomized Study with cross-over. J Clin Oncol 18:724–733
Paridaens RJ, Dirix LY, Beex LV et al (2008) Phase III study comparing exemestane with tamoxifen as first-line hormonal treatment of metastatic breast cancer in postmenopausal women: the European Organisation for Research and Treatment of Cancer Breast Cancer Cooperative Group. J Clin Oncol 26:4883–4890
Robert NJ, Saleh MN, Paul D et al (2011) Sunitinib plus paclitaxel versus bevacizumab plus paclitaxel for first-line treatment of patients with advanced breast cancer: a phase III, randomized, open-label trial. Clin Breast Cancer 11:82–92
Robert NJ, Dieras V, Glaspy J et al (2011) RIBBON-1: randomized, double-blind, placebo-controlled, phase III trial of chemotherapy with or without bevacizumab for first-line treatment of human epidermal growth factor receptor 2-negative, locally recurrent or metastatic breast cancer. J Clin Oncol 29:1252–1260
Rugo HS, Campone M, Amadori D et al (2013) A randomized, phase II, three-arm study of two schedules of ixabepilone or paclitaxel plus bevacizumab as first-line therapy for metastatic breast cancer. Breast Cancer Res Treat 139:411–419
Stockler MR, Harvey VJ, Francis PA et al (2011) Capecitabine versus classical cyclophosphamide, methotrexate, and fluorouracil as first-line chemotherapy for advanced breast cancer. J Clin Oncol 29:4498–4504
Vici P, Giotta F, Di Lauro L et al (2011) A multicenter phase II randomized trial of docetaxel/gemcitabine versus docetaxel/capecitabine as first-line treatment for advanced breast cancer: a Gruppo Oncologico Italia Meridionale study. Oncology 81:230–236
Zielinski C, Beslija S, Mrsic-Krmpotic Z et al (2005) Gemcitabine, epirubicin, and paclitaxel versus fluorouracil, epirubicin, and cyclophosphamide as first-line chemotherapy in metastatic breast cancer: a Central European Cooperative Oncology Group International, multicenter, prospective, randomized phase III trial. J Clin Oncol 23:1401–1408
Ghosn M, Aftimos P, Farhat FS et al (2011) A phase II randomized study comparing navelbine and capecitabine (Navcap) followed either by Navcap or by weekly docetaxel in the first-line treatment of HER-2/neu negative metastatic breast cancer. Med Oncol 28(Suppl 1):S142–S151
Jones AP, Haynes R, Sauerzapf V et al (2008) Travel time to hospital and treatment for breast, colon, rectum, lung, ovary and prostate cancer. Eur J Cancer 44:992–999
Yardley DA, Burris HA III, Spigel DR et al (2009) A phase II randomized crossover study of liposomal doxorubicin versus weekly docetaxel in the first-line treatment of women with metastatic breast cancer. Clin Breast Cancer 9:247–252
Acknowledgments
Financial support for the systematic literature review was provided by Novartis Farma—Italy to Ce.R.G.A.S. Bocconi University, Via Roentgen 1, Milan, Italy. The authors would like to acknowledge Maria Rosa Cappelletti and Laura Zanotti for the assistance in preparing the manuscript.
Conflict of interests
There are no financial or other interests with regard to the submitted manuscript that might be construed as a conflict of interest and authorization has been given to use any information conveyed by either personal communication or release of unpublished experimental data.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Generali, D., Venturini, S., Rognoni, C. et al. A network meta-analysis of everolimus plus exemestane versus chemotherapy in the first- and second-line treatment of estrogen receptor-positive metastatic breast cancer. Breast Cancer Res Treat 152, 95–117 (2015). https://doi.org/10.1007/s10549-015-3453-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10549-015-3453-9