Abstract
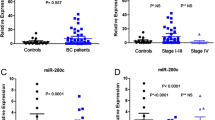
Trastuzumab and lapatinib are established treatments for patients with HER2 (human epidermal growth factor receptor 2)-positive breast cancer with different mechanisms of action. The focus of this study is to investigate, whether altered expression levels of potentially relevant microRNAs (miRs) in serum are associated with response to trastuzumab or lapatinib. Circulating miR-21, miR-210, and miR-373 were quantified with TaqMan MicroRNA assays in serum of 127 HER2-postive breast cancer patients before and after neoadjuvant therapy and in 19 healthy controls. Patients received chemotherapy combined with either trastuzumab or lapatinib within the prospectively randomized Geparquinto trial. The association between miR levels and pathological response (pCR) to therapy and type of therapy was examined. Serum levels of miR-21 (p = 5.04e-08, p = 1.43e-10), miR-210 (p = 0.00151, p = 1.6e-05), and miR-373 (p = 7.87e-06, p = 1.75e-07) were significantly higher in patients before and after chemotherapy than in healthy women. Concentrations of miR-21 (p = 5.73e-08), miR-210 (p = 0.000724), and miR-373 (p = 0.00209) increased further after chemotherapy. A significant association of higher serum levels of miR-373 with advanced clinical tumor stage could be detected (p < 0.002). An association of miR-21 levels before (p = 0.0091) and after (p = 0.037) chemotherapy with overall survival of the patients could be detected, independent of type of anti-HER2 therapy. No association of circulating miRs with pCR was found. Our findings demonstrate a specific influence of neoadjuvant therapy on the serum levels of miR-21, miR-210, and miR-373 in breast cancer patients together with a prognostic value of miR-21.



Similar content being viewed by others
References
Untch M, Loibl S, Bischoff J, Eidtmann H, Kaufmann M, Blohmer JU, Hilfrich J, Strumberg D, Fasching PA, Kreienberg R, Tesch H, Hanusch C, Gerber B, Rezai M, Jackisch C, Huober J, Kuhn T, Nekljudova V, von Minckwitz G, German Breast G, Arbeitsgemeinschaft Gynakologische Onkologie-Breast Study G (2012) Lapatinib versus trastuzumab in combination with neoadjuvant anthracycline-taxane-based chemotherapy (GeparQuinto, GBG 44): a randomised phase 3 trial. The lancet oncol 13(2):135–144. doi:10.1016/S1470-2045(11)70397-7
von Minckwitz G, Eidtmann H, Rezai M, Fasching PA, Tesch H, Eggemann H, Schrader I, Kittel K, Hanusch C, Kreienberg R, Solbach C, Gerber B, Jackisch C, Kunz G, Blohmer JU, Huober J, Hauschild M, Fehm T, Muller BM, Denkert C, Loibl S, Nekljudova V, Untch M, German Breast G, Arbeitsgemeinschaft Gynakologische Onkologie-Breast Study G (2012) Neoadjuvant chemotherapy and bevacizumab for HER2-negative breast cancer. The New Engl J Med 366(4):299–309
Buzdar AU, Ibrahim NK, Francis D, Booser DJ, Thomas ES, Theriault RL, Pusztai L, Green MC, Arun BK, Giordano SH, Cristofanilli M, Frye DK, Smith TL, Hunt KK, Singletary SE, Sahin AA, Ewer MS, Buchholz TA, Berry D, Hortobagyi GN (2005) Significantly higher pathologic complete remission rate after neoadjuvant therapy with trastuzumab, paclitaxel, and epirubicin chemotherapy: results of a randomized trial in human epidermal growth factor receptor 2-positive operable breast cancer. J Clin Oncol 23(16):3676–3685
Untch M, Rezai M, Loibl S, Fasching PA, Huober J, Tesch H, Bauerfeind I, Hilfrich J, Eidtmann H, Gerber B, Hanusch C, Kuhn T, du Bois A, Blohmer JU, Thomssen C, Dan Costa S, Jackisch C, Kaufmann M, Mehta K, von Minckwitz G (2010) Neoadjuvant treatment with Trastuzumab in HER2-positive breast cancer: results from the geparquattro study. J Clin Oncol 28(12):2024–2031
Untch M, Fasching PA, Konecny GE, Hasmuller S, Lebeau A, Kreienberg R, Camara O, Muller V, du Bois A, Kuhn T, Stickeler E, Harbeck N, Hoss C, Kahlert S, Beck T, Fett W, Mehta KM, von Minckwitz G, Loibl S (2011) Pathologic complete response after neoadjuvant chemotherapy plus trastuzumab predicts favorable survival in human epidermal growth factor receptor 2-overexpressing breast cancer: results from the TECHNO trial of the AGO and GBG study groups. J Clin Oncol 29(25):3351–3357
Figueroa-Magalhaes MC, Jelovac D, Connolly RM, Wolff AC (2014) Treatment of HER2-positive breast cancer. Breast 23(2):128–136
Schwarzenbach H, Nishida N, Calin GA, Pantel K (2014) Clinical relevance of circulating cell-free microRNAs in cancer. Nat Rev Clin Oncol 11(3):145–156
Kim VN (2005) MicroRNA biogenesis: coordinated cropping and dicing. Nat Rev Mol Cell Biol 6(5):376–385
Krol J, Loedige I, Filipowicz W (2010) The widespread regulation of microRNA biogenesis, function and decay. Nat Rev Genet 11(9):597–610
Heneghan HM, Miller N, Lowery AJ, Sweeney KJ, Kerin MJ (2009) MicroRNAs as novel biomarkers for breast cancer. J Oncol 2009:950201
Cortez MA, Calin GA (2009) MicroRNA identification in plasma and serum: a new tool to diagnose and monitor diseases. Expert Opin Biol Ther 9(6):703–711
Blenkiron C, Goldstein LD, Thorne NP, Spiteri I, Chin SF, Dunning MJ, Barbosa-Morais NL, Teschendorff AE, Green AR, Ellis IO, Tavare S, Caldas C, Miska EA (2007) MicroRNA expression profiling of human breast cancer identifies new markers of tumor subtype. Genome Biol 8(10):R214
Liu K, Li G, Fan C, Zhou X, Wu B, Li J (2011) Increased expression of microRNA-21 and its association with chemotherapeutic response in human colorectal cancer. J Int Med Res 39(6):2288–2295
Jung EJ, Santarpia L, Kim J, Esteva FJ, Moretti E, Buzdar AU, Di Leo A, Le XF, Bast RC Jr, Park ST, Pusztai L, Calin GA (2012) Plasma microRNA 210 levels correlate with sensitivity to trastuzumab and tumor presence in breast cancer patients. Cancer 118(10):2603–2614
Eichelser C, Flesch-Janys D, Chang-Claude J, Pantel K, Schwarzenbach H (2013) Deregulated serum concentrations of circulating cell-free microRNAs miR-17, miR-34a, miR-155, and miR-373 in human breast cancer development and progression. Clin Chem 59(10):1489–1496
Hayes DF, Ethier S, Lippman ME (2006) New guidelines for reporting of tumor marker studies in breast cancer research and treatment: REMARK. Breast Cancer Res Treat 100(2):237–238
McShane LM, Altman DG, Sauerbrei W, Taube SE, Gion M, Clark GM (2005) Reporting recommendations for tumor marker prognostic studies (REMARK). J Natl Cancer Inst 97(16):1180–1184
van Schooneveld E, Wouters MC, Van der Auwera I, Peeters DJ, Wildiers H, Van Dam PA, Vergote I, Vermeulen PB, Dirix LY, Van Laere SJ (2012) Expression profiling of cancerous and normal breast tissues identifies microRNAs that are differentially expressed in serum from patients with (metastatic) breast cancer and healthy volunteers. Breast Cancer Res 14(1):R34
Hu Z, Dong J, Wang LE, Ma H, Liu J, Zhao Y, Tang J, Chen X, Dai J, Wei Q, Zhang C, Shen H (2012) Serum microRNA profiling and breast cancer risk: the use of miR-484/191 as endogenous controls. Carcinogenesis 33(4):828–834
Lawrie CH, Gal S, Dunlop HM, Pushkaran B, Liggins AP, Pulford K, Banham AH, Pezzella F, Boultwood J, Wainscoat JS, Hatton CS, Harris AL (2008) Detection of elevated levels of tumour-associated microRNAs in serum of patients with diffuse large B-cell lymphoma. Br J Haematol 141(5):672–675
Wang B, Zhang Q (2012) The expression and clinical significance of circulating microRNA-21 in serum of five solid tumors. J Cancer Res Clin Oncol 138(10):1659–1666
Han M, Liu M, Wang Y, Mo Z, Bi X, Liu Z, Fan Y, Chen X, Wu C (2012) Re-expression of miR-21 contributes to migration and invasion by inducing epithelial-mesenchymal transition consistent with cancer stem cell characteristics in MCF-7 cells. Mol Cell Biochem 363(1–2):427–436
Anfossi S, Giordano A, Gao H, Cohen EN, Tin S, Wu Q, Garza RJ, Debeb BG, Alvarez RH, Valero V, Hortobagyi GN, Calin GA, Ueno NT, Woodward WA, Reuben JM (2014) High Serum miR-19a levels are associated with inflammatory breast cancer and are predictive of favorable clinical outcome in patients with metastatic HER2(+) inflammatory breast cancer. PLoS ONE 9(1):e83113
Eto K, Iwatsuki M, Watanabe M, Ida S, Ishimoto T, Iwagami S, Baba Y, Sakamoto Y, Miyamoto Y, Yoshida N, Baba H (2014) The MicroRNA-21/PTEN pathway regulates the sensitivity of HER2-positive gastric cancer cells to Trastuzumab. Ann Surg Oncol 21(1):343–350
Tanaka K, Miyata H, Yamasaki M, Sugimura K, Takahashi T, Kurokawa Y, Nakajima K, Takiguchi S, Mori M, Doki Y (2013) Circulating miR-200c levels significantly predict response to chemotherapy and prognosis of patients undergoing neoadjuvant chemotherapy for esophageal cancer. Ann Surg Oncol 20(Suppl 3):S607–S615
Drebber U, Lay M, Wedemeyer I, Vallbohmer D, Bollschweiler E, Brabender J, Monig SP, Holscher AH, Dienes HP, Odenthal M (2011) Altered levels of the onco-microRNA 21 and the tumor-supressor microRNAs 143 and 145 in advanced rectal cancer indicate successful neoadjuvant chemoradiotherapy. Int J Oncol 39(2):409–415
Huang Q, Gumireddy K, Schrier M, le Sage C, Nagel R, Nair S, Egan DA, Li A, Huang G, Klein-Szanto AJ, Gimotty PA, Katsaros D, Coukos G, Zhang L, Pure E, Agami R (2008) The microRNAs miR-373 and miR-520c promote tumour invasion and metastasis. Nat Cell Biol 10(2):202–210
Acknowledgments
This study was supported by the Deutsche Krebshilfe e.V (199232) and ERC (European Research Council) Advanced Investigator Grant (ERC-2010 AsG 20100317 DISSECT).
Conflict of interest
No potential conflicts of interest were disclosed by all authors.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Fig. S1
Diagram showing the number of participants in the GeparQuinto study with HER2 positive tumors analyzed (PPT 102 kb)
Fig. S2
Comparison of the three miRs between trastuzumab and lapatinib before and after therapy. All p-values were calculated using two-sided Wilcoxon tests (PPTX 79 kb)
Table S1
(DOCX 15 kb)
Table S2
(DOCX 18 kb)
Table S3
(DOCX 18 kb)
Rights and permissions
About this article
Cite this article
Müller, V., Gade, S., Steinbach, B. et al. Changes in serum levels of miR-21, miR-210, and miR-373 in HER2-positive breast cancer patients undergoing neoadjuvant therapy: a translational research project within the Geparquinto trial. Breast Cancer Res Treat 147, 61–68 (2014). https://doi.org/10.1007/s10549-014-3079-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10549-014-3079-3