Abstract
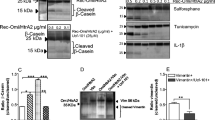
Neuronal cell death after traumatic brain injury, Alzheimer’s disease and ischemic stroke may in part be mediated through endoplasmic reticulum (ER) stress and unfolded protein response (UPR). UPR results in induction of molecular chaperone GRP78 and the ER-resident caspase-12, whose activation has been proposed to be mediated by calpain and caspase processing, although their relative contribution remains unclear. In this study we induced ER stress with thapsigargin (TG), and determined the activation profile of calpain-2, caspase-3, caspase-7, and caspase-12 by analyses of protein levels, corresponding substrates and breakdown products (BDP). Specific calpain and caspase activity was assessed by analysis of αII-spectrin BDP of 145 kDa (SBDP145), BDP of 150 kDa (SBDP150) and BDP of 120 kDa (SBDP120). Decrease in pro-calpain-2 protein and increased SBDP145 levels by 3 h after TG treatment indicated early calpain activity. Active caspase-7 (p20) increase occurred after 8 h, followed by concomitant up-regulation of active caspase-3 and SBDP120 after 24 h. In vitro digestion experiments supported that SBDP120 was exclusively generated by active caspase-3 and validated that kinectin and co-chaperone p23 were calpain and caspase-7 substrates, respectively. Pro-caspase-12 protein processing by the specific action of calpain and caspase-3/7 was observed in a time-dependent manner. N-terminal pro-domain processing of pro-caspase-12 by calpain generated a 38 kDa fragment, while caspase-3/7 generated a 35 kDa fragment. Antibody developed specifically against the caspase-3/7 C-terminal cleavage site D341 detected the presence of large subunit (p20) containing 23 kDa fragment that increased after 24 h of TG treatment. Significant caspase-12 enzyme activity was only detected after 24 h of TG treatment and was completely inhibited by caspase 3/7 inhibitor DEVD-fmk and partially by calpain inhibitor SNJ-1945. ER-stress-induced cell death pathway in TG-treated PC12 cells was characterized by up-regulation of GRP-78 and processing and activation of caspase-12 by the orchestrated proteolytic activity of calpain-2 and caspase-3/7.










Similar content being viewed by others
Abbreviations
- ER:
-
Endoplasmic reticulum
- UPR:
-
Unfolded protein response
- GRP-78:
-
Glucose responsive protein of 78 kDa
- TG:
-
Thapsigargin
- BDP:
-
Breakdown product
- TBI:
-
Traumatic brain injury
References
Ellgaard L, Helenius A (2003) Quality control in the endoplasmic reticulum. Nat Rev Mol Cell Biol 4:181–191
Freeze HH (2007) Congenital disorders of glycosylation: CDG-I, CDG-II, and beyond. Curr Mol Med 7:389–396
Zhang K, Kaufman RJ (2006) The unfolded protein response: a stress signaling pathway critical for health and disease. Neurology 66:S102–S109
Zhao L, Ackerman SL (2006) Endoplasmic reticulum stress in health and disease. Curr Opin Cell Biol 18:444–452
Lindholm D, Wootz H, Korhonen L (2006) ER stress and neurodegenerative diseases. Cell Death Differ 13:385–392
Larner SF, Hayes RL, Wang KK (2006) Unfolded protein response after neurotrauma. J Neurotrauma 23:807–829
Truettner JS, Hu B, Alonso OF, Bramlett HM, Kokame K, Dietrich WD (2007) Subcellular stress response after traumatic brain injury. J Neurotrauma 24:599–612
Mori K (2000) Tripartite management of unfolded proteins in the endoplasmic reticulum. Cell 101:451–454
Momoi T (2004) Caspases involved in ER stress-mediated cell death. J Chem Neuroanat 28:101–105
Tan Y, Dourdin N, Wu C, De Veyra T, Elce JS, Greer PA (2006) Ubiquitous calpains promote caspase-12 and JNK activation during endoplasmic reticulum stress-induced apoptosis. J Biol Chem 281:16016–16024
Masud A, Mohapatra A, Lakhani SA, Ferrandino A, Hakem R, Flavell RA (2007) Endoplasmic reticulum stress-induced death of mouse embryonic fibroblasts requires the intrinsic pathway of apoptosis. J Biol Chem 282:14132–14139
Rao RV, Hermel E, Castro-Obregon S et al (2001) Coupling endoplasmic reticulum stress to the cell death program. Mechanism of caspase activation. J Biol Chem 276:33869–33874
Nakagawa T, Yuan J (2000) Cross-talk between two cysteine protease families. Activation of caspase-12 by calpain in apoptosis. J Cell Biol 150:887–894
Saleh M, Vaillancourt JP, Graham RK et al (2004) Differential modulation of endotoxin responsiveness by human caspase-12 polymorphisms. Nature 429:75–79
Sanges D, Marigo V (2006) Cross-talk between two apoptotic pathways activated by endoplasmic reticulum stress: differential contribution of caspase-12 and AIF. Apoptosis 11:1629–1641
Shirasaki Y, Miyashita H, Yamaguchi M, Inoue J, Nakamura M (2005) Exploration of orally available calpain inhibitors: peptidyl alpha-ketoamides containing an amphiphile at P3 site. Bioorg Med Chem 13:4473–4484
Cryns V, Yuan J (1998) Proteases to die for. Genes Dev 12:1551–1570
Nath R, Raser KJ, Stafford D et al (1996) Non-erythroid alpha-spectrin breakdown by calpain and interleukin 1 beta-converting-enzyme-like protease(s) in apoptotic cells: contributory roles of both protease families in neuronal apoptosis. Biochem J 319(Pt 3):683–690
Newcomb JK, Kampfl A, Posmantur RM et al (1997) Immunohistochemical study of calpain-mediated breakdown products to alpha-spectrin following controlled cortical impact injury in the rat. J Neurotrauma 14:369–383
Newcomb-Fernandez JK, Zhao X, Pike BR et al (2001) Concurrent assessment of calpain and caspase-3 activation after oxygen-glucose deprivation in primary septo-hippocampal cultures. J Cereb Blood Flow Metab 21:1281–1294
Nath R, Huggins M, Glantz SB et al (2000) Development and characterization of antibodies specific to caspase-3-produced alpha II-spectrin 120 kDa breakdown product: marker for neuronal apoptosis. Neurochem Int 37:351–361
Zhang Z, Larner SF, Liu MC, Zheng W, Hayes RL, Wang KK (2009) Multiple alphaII-spectrin breakdown products distinguish calpain and caspase dominated necrotic and apoptotic cell death pathways. Apoptosis 14(11):1289–1298
Harriman JF, Liu XL, Aleo MD, Machaca K, Schnellmann RG (2002) Endoplasmic reticulum Ca(2+) signaling and calpains mediate renal cell death. Cell Death Differ 9:734–741
Walsh JG, Cullen SP, Sheridan C, Luthi AU, Gerner C, Martin SJ (2008) Executioner caspase-3 and caspase-7 are functionally distinct proteases. Proc Natl Acad Sci USA 105:12815–12819
Machleidt T, Geller P, Schwandner R, Scherer G, Kronke M (1998) Caspase 7-induced cleavage of kinectin in apoptotic cells. FEBS Lett 436:51–54
Chan SL, Mattson MP (1999) Caspase and calpain substrates: roles in synaptic plasticity and cell death. J Neurosci Res 58:167–190
Wang KK (2000) Calpain and caspase: can you tell the difference? Trends Neurosci 23:20–26
Harwood SM, Yaqoob MM, Allen DA (2005) Caspase and calpain function in cell death: bridging the gap between apoptosis and necrosis. Ann Clin Biochem 42:415–431
Wang KK, Posmantur R, Nath R et al (1998) Simultaneous degradation of alphaII- and betaII-spectrin by caspase 3 (CPP32) in apoptotic cells. J Biol Chem 273:22490–22497
Tran H, Pankov R, Tran SD, Hampton B, Burgess WH, Yamada KM (2002) Integrin clustering induces kinectin accumulation. J Cell Sci 115:2031–2040
Kim I, Xu W, Reed JC (2008) Cell death and endoplasmic reticulum stress: disease relevance and therapeutic opportunities. Nat Rev Drug Discov 7:1013–1030
Yoshida H (2007) ER stress and diseases. FEBS J 274:630–658
Truettner JS, Hu K, Liu CL, Dietrich WD, Hu B (2009) Subcellular stress response and induction of molecular chaperones and folding proteins after transient global ischemia in rats. Brain Res 1249:9–18
Rao RV, Castro-Obregon S, Frankowski H et al (2002) Coupling endoplasmic reticulum stress to the cell death program. An Apaf-1-independent intrinsic pathway. J Biol Chem 277:21836–21842
Voccoli V, Mazzoni F, Garcia-Gil M, Colombaioni L (2007) Serum-withdrawal-dependent apoptosis of hippocampal neuroblasts involves Ca++ release by endoplasmic reticulum and caspase-12 activation. Brain Res 1147:1–11
Muruganandan S, Cribb AE (2006) Calpain-induced endoplasmic reticulum stress and cell death following cytotoxic damage to renal cells. Toxicol Sci 94:118–128
Hiroi T, Wei H, Hough C, Leeds P, Chuang DM (2005) Protracted lithium treatment protects against the ER stress elicited by thapsigargin in rat PC12 cells: roles of intracellular calcium, GRP78 and Bcl-2. Pharmacogenomics J 5:102–111
Shimoke K, Kishi S, Utsumi T et al (2005) NGF-induced phosphatidylinositol 3-kinase signaling pathway prevents thapsigargin-triggered ER stress-mediated apoptosis in PC12 cells. Neurosci Lett 389:124–128
Lee W, Kim DH, Boo JH, Kim YH, Park IS, Mook-Jung I (2005) ER stress-induced caspase-12 activation is inhibited by PKC in neuronal cells. Apoptosis 10:407–415
Korfali N, Ruchaud S, Loegering D et al (2004) Caspase-7 gene disruption reveals an involvement of the enzyme during the early stages of apoptosis. J Biol Chem 279:1030–1039
Jang M, Park BC, Lee AY et al (2007) Caspase-7 mediated cleavage of proteasome subunits during apoptosis. Biochem Biophys Res Commun 363:388–394
Stegh AH, Kesari S, Mahoney JE et al (2008) Bcl2L12-mediated inhibition of effector caspase-3 and caspase-7 via distinct mechanisms in glioblastoma. Proc Natl Acad Sci USA 105:10703–10708
Van de Craen M, Vandenabeele P, Declercq W et al (1997) Characterization of seven murine caspase family members. FEBS Lett 403:61–69
Hoppe V, Hoppe J (2004) Mutations dislocate caspase-12 from the endoplasmic reticulum to the cytosol. FEBS Lett 576:277–283
Korge P, Weiss JN (1999) Thapsigargin directly induces the mitochondrial permeability transition. Eur J Biochem 265:273–280
Baumgartner HK, Gerasimenko JV, Thorne C et al (2009) Calcium elevation in mitochondria is the main Ca2+ requirement for mitochondrial permeability transition pore (mPTP) opening. J Biol Chem 284:20796–20803
Morishima N, Nakanishi K, Takenouchi H, Shibata T, Yasuhiko Y (2002) An endoplasmic reticulum stress-specific caspase cascade in apoptosis. Cytochrome c-independent activation of caspase-9 by caspase-12. J Biol Chem 277:34287–34294
Mao W, Iwai C, Keng PC, Vulapalli R, Liang CS (2006) Norepinephrine-induced oxidative stress causes PC-12 cell apoptosis by both endoplasmic reticulum stress and mitochondrial intrinsic pathway: inhibition of phosphatidylinositol 3-kinase survival pathway. Am J Physiol Cell Physiol 290:C1373–C1384
Fujita E, Kouroku Y, Jimbo A, Isoai A, Maruyama K, Momoi T (2002) Caspase-12 processing and fragment translocation into nuclei of tunicamycin-treated cells. Cell Death Differ 9:1108–1114
Acknowledgements
This work was supported by NIH Grant R01NS051431 to R. L. H. Dr. K. K. W. Wang and Dr. R. L. Hayes hold equity in Banyan Biomarkers, Inc. (Alachua, FL), a company commercializing the brain injury biomarker technology.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Martinez, J.A., Zhang, Z., Svetlov, S.I. et al. Calpain and caspase processing of caspase-12 contribute to the ER stress-induced cell death pathway in differentiated PC12 cells. Apoptosis 15, 1480–1493 (2010). https://doi.org/10.1007/s10495-010-0526-4
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10495-010-0526-4