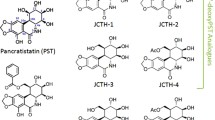
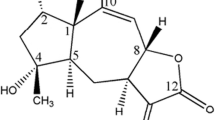
Abstract
The major hurdle in the fight against cancer is the non-specific nature of current treatments. The search for specific drugs that are non-cytotoxic to normal cells and can effectively target cancer cells has lead some researchers to investigate the potential anti-cancer activity of natural compounds. Some natural compounds, such as Taxol, have been shown to posses some anti-cancer potential. Pancratistatin (PST) is a natural compound that was isolated from the spider lily Pancratium littorale and shown to exhibit antineoplastic activity. The specificity of PST to cancer cells and the mechanism of PST’s action remain unknown. This study provides a detailed look at the effect of PST treatment on cancerous and normal cells. Our results indicate that PST induced apoptosis selectively in cancer cells and that the mitochondria may be the site of action of PST in cancer cells. A biochemical target available specifically in cancer cells may lead to the development of new and more effective cancer fighting agents.
Similar content being viewed by others
References
Hui W, Kavanagh JJ. Anticancer therapy targeting the apoptotic pathway. Lancet Oncol 2003; 4: 721–729.
Boose G, Stopper H. Genotoxicity of several clinically used topoisomerase II inhibitors. Toxicology Letters 2000; 116: 7–16.
Pandey S, Smith B, Walker PR, Sikorska M. Caspase-dependent and independent cell death in rat hepatoma 5123tc cells. Apoptosis 2000; 5: 265–275.
Green D, Kroemer G. The pathology of mitochondrial cell death. Science 2004; 305: 626–629.
Kerr, JF, Wyllie AH, Currie AR. Apoptosis: A basic biological phenomenon with wide-ranging implications in tissue kinetics. Br J Cancer 1972; 26: 239–257.
Corcoran GB, Fix L, Jones DP, et al. Apoptosis: Molecular control point in toxicity. Toxicol Appl Pharmacol 1994; 128: 169–181.
Kazi A, Hill R, Long T, Kuhn D, Turos E, Dou P. Novel N-thiolated β -lactam anti-biotics selectively induce apoptosis in human tumor and transformed, but not normal or nontransformed, cells. Biochem Pharacol 2004; 67: 365–374.
Yuan H, Mutaomba M, Prinz I, Gottlieb R. Differential processing of cytosolic and mitochondrial caspases. Mitochondrion 2001; 1: 61–69.
Orrenius S. Mitochondrial regulation of apoptotic cell death. Toxicol Lett 2004; 149: 19–23.
Don A, Hogg P. Mitochondria as cancer drug targets. Trends Mol Med 2004; 10: 8.
Kroemer G. Mitochondrial control of apoptosis: An introduction. Biochem Biophys Res Comm 2003; 302: 433–435.
Fantin VR, Leder P. F16, a mitochondriotoxic compound, trigger apoptosis or necrosis depending on the genetic background of the target carcinoma cell. Cancer Res 2004; 64: 329–336.
Modica-Naoolitano J, Singh K. Mitochondria as targets for detection and treatment of cancer. Exp Rev Mol Med 11 April, 2002; http://www-ermm.cbcu.cam.ac.uk/02004453h.htm.
Luduena RF, Roach MC, Prasad V, Pettit GR. Biochemical Pharmacol 1992; 43: 539.
Pandey S, Kekre N, McNulty J, Naderi J. Induction of apoptosis by pancratistatin specifically in cancer cells. Artificial cells, blood substitutes and biotechnology 2004; In Press
Pettit GR, Pettit GR III, Backhaus RA, Boyd MR, Meerow AW. Antineoplastic Agents 256. Cell Growth Inhibitory Isocarbostyrils from Hymenocallis. J Nat Prod 1993; 56: 1682.
Naderi J, Hung M, Pandey S. Oxidative stress-induced apoptosis in dividing fibroblasts involves activation of p38 MAP kinase and over-expression of Bax: Resistance of quiescent cells to oxidative stress. Apoptosis 2003; 8: 91–100.
Siraki AG, Pourahmad J, Chan TS, Khon S, O’Brien PJ. Endogenous and endobiotic reactive oxygen species formation by isolated hepatocytes. Free Radical Bio Med 2002; 32: 2–10.
Olichon A, Baricault L, Gas N, et al. Loss of OPA1 perturbates the mitochondrial inner membrane structure and integrity, leading to cytochrome c release and apoptosis. J Biol Chem 2003; 278: 7743–7746.
Li N, Ragheb K, Lawler G, Sturgis J, Rajwa B, Melendez JA, Robinson JP. Mitochondrial complex-I inhibitor rotenone induces apoptosis through enhancing mitochondrial reactive oxygen species production. J Biol Chem 2003; 278: 8516–8525.
Ashkenazi A, Dixit VM. Death receptors; Signaling and modulation. Science 1998; 281: 1305–1308.
McCarthy S, Somayajulu M, Sikorska M, Borowy-Borowski H, Pandey S. Paraquat induces oxidative stress and neuronal cell death; neuroprotection by water-soluble Coenzyme Q10. Toxicol Appl Pharmacol 2004; article in press.
Fleury C, Mignotte B, Vayssiere JL. Mitochondrial reactive oxygen species in cell death signaling. Biochemie 2002; 84: 131–141.
Fernadez-Chenca JC. Redox regulation and signaling lipids in mitochondrial apoptosis. Biochem Biophys Res Comm 2003; 304: 471–479.
Duchen, MR. Roles of mitochondria in health and disease. Diabetes 2004; 53: S96–102.
Green DR, Reed JC. Mitochondria and apoptosis. Science 1998; 281: 1309–1312.
Villa AM, Doglia SM. Mitochondria in tumor cells studied by laser scanning confocal microcopy. J Biomed Opt 2004; 9: 385–394.
Polyak K, Li Y, Zhu H, et al. Somatic mutations of the mitochondrial genome in human colorectal tumors. Nat Genet 1998; 20: 291–293.
Sugiura T, Nagano Y, Inoue T, Hirotani K. A novel mitochondrial C1-tetrahydrofolate synthetase is up-regulated in human colon adenocarcinoma. Biochem Biophys Res Comm 2004; 315: 204–211.
Parlo R, Coleman P. Enhanced rate of citrate export from cholesterol-rich hepatoma mitochondria. J Biol Chem 1984; 259: 9997–10003.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
McLachlan, A., Kekre, N., McNulty, J. et al. Pancratistatin: A natural anti-cancer compound that targets mitochondria specifically in cancer cells to induce apoptosis. Apoptosis 10, 619–630 (2005). https://doi.org/10.1007/s10495-005-1896-x
Issue Date:
DOI: https://doi.org/10.1007/s10495-005-1896-x