Abstract
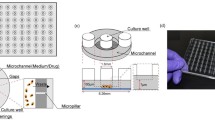
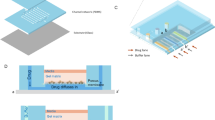
For personalized screening and therapeutic inventions across many diseases, the drug–dose response for an individual patient is a major unmet need. In this work, we applied a direct strategy to generate a static microwell array for cell culture with uniform cell seeding and to create the desired chemical concentration gradient into this cell array. It is a simple, novel and easily operable device for a high-throughput drug–dose response experiment that complies with the procedures of cell-based drug screening. Only two repeated operating steps suffice for the entire cell-based drug test—an injection of cells or drug into the flow channel and then injection of air flow into the flow channel. This device comprises two PDMS layers: one side forms an air chamber; the other side forms the liquid channel with embedded cavities as the cells culture and reaction region. The concentration of the drug decreases exponentially along the microwell array; the range of the concentration gradient is varied with the rate of air flow, the volume of the drug plug and the initial concentration of the drug. Small variations of concentration were accessed across varied ranges; the IC50 value of DOX in the MDA-MB-231 cell line was thus utilized on this device, precisely and quickly. The IC50 value calculated in this work is consistent with the range published elsewhere. With this device, hundreds of data points per compound drug screening can be tested in one experiment, which will be an essential key to determine customized drug dosage and to make possible personalized medicine.





Similar content being viewed by others
References
Abate AR, Hung T, Mary P, Agresti JJ, Weitz DA (2010) High-throughput injection with microfluidics using picoinjectors. Proc Natl Acad Sci 107(45):19163–19166
Abe Y, Kamiya K, Osaki T, Sasaki H, Kawano R, Miki N, Takeuchi S (2015) Nonlinear concentration gradients regulated by the width of channels for observation of half maximal inhibitory concentration (IC 50) of transporter proteins. Analyst 140(16):5557–5562
Aykul S, Martinez-Hackert E (2016) Determination of half-maximal inhibitory concentration using biosensor-based protein interaction analysis. Anal Biochem 508:97–103
Bao L, Rezk AR, Yeo LY, Zhang X (2015) Highly ordered arrays of femtoliter surface droplets. Small 11(37):4850–4855
Bithi SS, Wang WS, Sun M, Blawzdziewicz J, Vanapalli SA (2014) Coalescing drops in microfluidic parking networks: a multifunctional platform for drop-based microfluidics. Biomicrofluidics 8(3):034118
Burdick JA, Khademhosseini A, Langer R (2004) Fabrication of gradient hydrogels using a microfluidics/photopolymerization process. Langmuir 20(13):5153–5156
Chang CW, Cheng YJ, Tu M, Chen YH, Peng CC, Liao WH, Tung YC (2014) A polydimethylsiloxane–polycarbonate hybrid microfluidic device capable of generating perpendicular chemical and oxygen gradients for cell culture studies. Lab Chip 14(19):3762–3772
Chen CY, Wo AM, Jong DS (2012) A microfluidic concentration generator for dose-response assays on ion channel pharmacology. Lab Chip 12(4):794–801
Dertinger SK, Chiu DT, Jeon NL, Whitesides GM (2001) Generation of gradients having complex shapes using microfluidic networks. Anal Chem 73(6):1240–1246
Dittrich PS, Manz A (2006) Lab-on-a-chip: microfluidics in drug discovery. Nat Rev Drug Discovery 5(3):210–218
Dressler OJ, Maceiczyk RM, Chang S-I (2014) Droplet-based microfluidics enabling impact on drug discovery. J Biomol Screen 19(4):483–496
Eddings MA, Gale BK (2006) A PDMS-based gas permeation pump for on-chip fluid handling in microfluidic devices. J Micromech Microeng 16(11):2396
Eribol P, Uguz A, Ulgen K (2016) Screening applications in drug discovery based on microfluidic technology. Biomicrofluidics 10(1):011502
Fujii T (2002) PDMS-based microfluidic devices for biomedical applications. Microelectron Eng 61:907–914
Gao Y, Sun J, Lin WH, Webb DJ, Li D (2012) A compact microfluidic gradient generator using passive pumping. Microfluid Nanofluid 12(6):887–895
Ginsburg GS, McCarthy JJ (2001) Personalized medicine: revolutionizing drug discovery and patient care. Trends Biotechnol 19(12):491–496
Grishagin IV (2015) Automatic cell counting with ImageJ. Anal Biochem 473:63–65
Holden MA, Kumar S, Castellana ET, Beskok A, Cremer PS (2003) Generating fixed concentration arrays in a microfluidic device. Sens Actuators B: Chem 92(1):199–207
Huang PH, Chan CY, Li P, Nama N, Xie Y, Wei CH, Chen Y, Ahmed D, Huang TJ (2015) A spatiotemporally controllable chemical gradient generator via acoustically oscillating sharp-edge structures. Lab Chip 15(21):4166–4176
Hung PJ, Lee PJ, Sabounchi P, Lin R, Lee LP (2005) Continuous perfusion microfluidic cell culture array for high-throughput cell-based assays. Biotechnol Bioeng 89(1):1–8
Iwasa J, Ochi M, Uchio Y, Katsube K, Adachi N, Kawasaki K (2003) Effects of cell density on proliferation and matrix synthesis of chondrocytes embedded in atelocollagen gel. Artif Organs 27(3):249–255
Jeon NL, Dertinger SK, Chiu DT, Choi IS, Stroock AD, Whitesides GM (2000) Generation of solution and surface gradients using microfluidic systems. Langmuir 16(22):8311–8316
Jonas O, Landry HM, Fuller JE, Santini JT, Baselga J, Tepper RI, Cima MJ, Langer R (2015) An implantable microdevice to perform high-throughput in vivo drug sensitivity testing in tumors. Sci Transl Med 7(284):284ra257
Kalet BT (2007) Doxorubicin and its formaldehyde conjugates (Doctoral dissertation, University of Colorado at Boulder)
Kawai H, Minamiya Y, Kitamura M, Matsuzaki I, Hashimoto M, Suzuki H, Abo S (1997) Direct measurement of doxorubicin concentration in the intact, living single cancer cell during hyperthermia. Cancer 79(2):214–219
Kim D, Lokuta MA, Huttenlocher A, Beebe DJ (2009) Selective and tunable gradient device for cell culture and chemotaxis study. Lab Chip 9(12):1797–1800
Kim S, Kim HJ, Jeon NL (2010) Biological applications of microfluidic gradient devices. Integr Biol 2(11–12):584–603
Lamberti A, Marasso SL, Cocuzza M (2014) PDMS membranes with tunable gas permeability for microfluidic applications. Rsc Adv 4(106):61415–61419
Lee DW, Lee M-Y, Ku B, Nam D-H (2015) Automatic 3d cell analysis in high-throughput microarray using micropillar and microwell chips. J Biomol Screen. doi:10.1177/1087057115597635
Li X, Zhang X, Zhao S, Wang J, Liu G, Du Y (2014) Micro-scaffold array chip for upgrading cell-based high-throughput drug testing to 3D using benchtop equipment. Lab Chip 14(3):471–481
Neuži P, Giselbrecht S, Länge K, Huang TJ, Manz A (2012) Revisiting lab-on-a-chip technology for drug discovery. Nat Rev Drug Discov 11(8):620–632
Ng EX, Miller MA, Jing T, Lauffenburger DA, Chen C-H (2015) Low-volume multiplexed proteolytic activity assay and inhibitor analysis through a pico-injector array. Lab Chip 15(4):1153–1159
Pompano RR, Liu W, Du W, Ismagilov RF (2011) Microfluidics using spatially defined arrays of droplets in one, two, and three dimensions. Annu Rev Anal Chem 4:59–81
Saini KS, Hajrah NH (2015) Toxicogenomics–getting us a step closer towards personalized medicine. J Investig Genomics 2(3):00024
Sawant S, Shegokar R (2014) Cancer research and therapy: where are we today? Int J Cancer Therapy Oncol 2(4):02048
Somaweera H, Ibraguimov A, Pappas D (2016) A review of chemical gradient systems for cell analysis. Anal Chim Acta 907:7–17
Sun M, Vanapalli SA (2013) Generation of chemical concentration gradients in mobile droplet arrays via fragmentation of long immiscible diluting plugs. Anal Chem 85(4):2044–2048
Sun M, Bithi SS, Vanapalli SA (2011) Microfluidic static droplet arrays with tuneable gradients in material composition. Lab Chip 11(23):3949–3952
Tassone P, Tagliaferri P, Perricelli A, Blotta S, Quaresima B, Martelli M, Goel A, Barbieri V, Costanzo F, Boland C (2003) BRCA1 expression modulates chemosensitivity of BRCA1-defective HCC1937 human breast cancer cells. Br J Cancer 88(8):1285–1291
Taylor G (1953) Dispersion of soluble matter in solvent flowing slowly through a tube. In: Proceedings of the royal society of London a: mathematical, physical and engineering sciences. The Royal Society 1137:186–203
Tegze B, Szállási Z, Haltrich I, Pénzváltó Z, Tóth Z, Likó I, Győrffy B (2012) Parallel evolution under chemotherapy pressure in 29 breast cancer cell lines results in dissimilar mechanisms of resistance. PLoS ONE 7(2):e30804
Toh YC, Lim TC, Tai D, Xiao G, van Noort D, Yu H (2009) A microfluidic 3D hepatocyte chip for drug toxicity testing. Lab Chip 9(14):2026–2035
Toh AG, Wang Z, Yang C, Nguyen N-T (2014) Engineering microfluidic concentration gradient generators for biological applications. Microfluid Nanofluid 16(1–2):1–18
Vickerman V, Blundo J, Chung S, Kamm R (2008) Design, fabrication and implementation of a novel multi-parameter control microfluidic platform for three-dimensional cell culture and real-time imaging. Lab Chip 8(9):1468–1477
Xu BY, Hu SW, Qian GS, Xu JJ, Chen HY (2013) A novel microfluidic platform with stable concentration gradient for on chip cell culture and screening assays. Lab Chip 13(18):3714–3720
Xu Z, Huang X, Wang P, Wang H, Weitz DA (2016) Optimization and development of a universal flow-based microfluidic gradient generator. Microfluid Nanofluid 20(6):1–10
Yang CG, Liu YH, Di YQ, Xu ZR (2015) Generation of two-dimensional concentration-gradient droplet arrays on a two-layer chip for screening of protein crystallization conditions. Microfluid Nanofluid 18(3):493–501
Yeh CH, Chen CH, Lin YC (2011) Use of a gradient-generating microfluidic device to rapidly determine a suitable glucose concentration for cell viability test. Microfluid Nanofluid 10(5):1011–1018
Yeh SI, Fang WF, Huang CJ, Wang TM, Yang JT (2016) The visual colorimetric detection of multi-nucleotide polymorphisms on a pneumatic droplet manipulation platform. J Vis Exp 115:e54424–e54424
Acknowledgements
Ministry of Science and Technology of Taiwan provided financial support of this research under contract MOST 103-2221-E-002-097-MY3.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Yeh, S.I., Hau, C.C., Huang, C.J. et al. Development of a simple static microwell array with uniform cell seeding and a chemical concentration gradient. Microfluid Nanofluid 21, 80 (2017). https://doi.org/10.1007/s10404-017-1921-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10404-017-1921-8