Abstract
Background
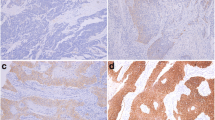
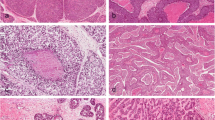
Esophageal squamous intraepithelial neoplasia (ESIN) as a precursor for invasive carcinoma is classified into low-grade IN (LIN) and high-grade IN (HIN), the latter including carcinoma in situ (CIS). Increasing grades of ESIN are associated with increasing risk of invasive carcinoma, but differentiation among LIN, HIN, and CIS is subjective and challenging. As p63, a member of the p53 family, is known to regulate differentiation and proliferation in epithelial progenitor cells, its expression, along with that of cytokeratin (CK) 14 (basal squamous marker) and Ki-67 (proliferation marker), might be of diagnostic assistance.
Methods
Immunohistochemical staining was performed on endoscopically resected specimens from 69 patients (73 lesions) with ESIN between 2008 and 2010. Specimens having no atypical cells distant from ESIN were defined as negative for IN (NIN).
Results
There were 13 LIN, 29 HIN, and 31 CIS lesions. The size of CIS (mean 20.1 mm) was significantly larger than that of HIN (14.3 mm) or LIN (10.0 mm). Immunolabeling scores (ILS) for p63, p53, CK14, and Ki-67 showed stepwise increase with ESIN progression (NIN vs. LIN and LIN vs. HIN, P < 0.05–0.0001; HIN vs. CIS, P < 0.05–0.001). Mean total ILSs of all four markers were 2.0 for NIN, 4.3 for LIN, 7.4 for HIN, and 9.1 for CIS (P < 0.0001). A cutoff value for total ILS of ≤5 versus ≥6 demonstrated highest sensitivity (96.0%) and specificity (85.7%) in distinguishing HIN from LIN.
Conclusions
This study suggests that p63 and p53 are coordinately upregulated in esophageal squamous carcinogenesis, and assessment of their expression might provide a useful adjunct tool for differential diagnosis of ESIN.





Similar content being viewed by others
References
Parkin DM, Bray F, Ferlay J, Pisani P. Global cancer statistics, 2002. CA Cancer J Clin. 2005;55:10–74.
Takubo K, Aida J, Sawabe M, Kurosumi M, Arima M, Fujishiro M, Arai T. Early squamous cell carcinoma of the oesophagus: the Japanese viewpoint. Histopathology. 2007;51:733–42.
Dawsey SM, Lewin KJ, Wang GQ, Liu FS, Nieberg RK, Yu Y, Li JY, Blot WJ, Li B, Taylor PR. Squamous esophageal histology and subsequent risk of squamous cell carcinoma of the esophagus. A prospective follow-up study from Linxian, China. Cancer. 1994;74:1686–92.
Wang GQ, Abnet CC, Shen Q, Lewin KJ, Sun XD, Roth MJ, Qiao YL, Mark SD, Dong ZW, Taylor PR, Dawsey SM. Histological precursors of oesophageal squamous cell carcinoma: results from a 13 year prospective follow up study in a high risk population. Gut. 2005;54:187–92.
Gabbert HE, Shimoda T, Hainaut P, Nakamura Y, Field JK, Inoue H. Squamous cell carcinoma of the oesophagus. In: Hamilton SR, Aaltonen LA, editors. World Health Organization classification of tumours. Pathology and genetics. Tumours of the digestive system. Lyon: IARC Press; 2000. p. 8–19.
Schlemper RJ, Dawsey SM, Itabashi M, Iwashita A, Kato Y, Koike M, Lewin KJ, Riddell RH, Shimoda T, Sipponen P, Stolte M, Watanabe H. Differences in diagnostic criteria for esophageal squamous cell carcinoma between Japanese and Western pathologists. Cancer. 2000;88:996–1006.
Levine AJ, Wu MC, Chang A, Silver A, Attiyeh EF, Lin J, Epstein CB. The spectrum of mutations at the p53 locus. Evidence for tissue-specific mutagenesis, selection of mutant alleles, and a “gain of function” phenotype. Ann N Y Acad Sci. 1995;768:111–28.
Polyak K, Xia Y, Zweier JL, Kinzler KW, Vogelstein B. A model for p53-induced apoptosis. Nature. 1997;389:300–5.
Irwin MS, Kaelin WG Jr. Role of the newer p53 family proteins in malignancy. Apoptosis. 2001;6:17–29.
Ratovitski EA, Patturajan M, Hibi K, Trink B, Yamaguchi K, Sidransky D. p53 associates with and targets delta Np63 into a protein degradation pathway. Proc Natl Acad Sci USA. 2001;98:1817–22.
Hibi K, Trink B, Patturajan M, Westra WH, Caballero OL, Hill DE, Ratovitski EA, Jen J, Sidransky D. AIS is an oncogene amplified in squamous cell carcinoma. Proc Natl Acad Sci USA. 2000;97:5462–7.
Chu PG, Weiss LM. Keratin expression in human tissues and neoplasms. Histopathology. 2002;40:403–9.
Moll R, Franke WW, Schiller DL, Geiger B, Krepler R. The catalog of human cytokeratins: patterns of expression in normal epithelia, tumors and cultured cells. Cell. 1982;31:11–24.
Hesse M, Magin TM, Weber K. Genes for intermediate filament proteins and the draft sequence of the human genome: novel keratin genes and a surprisingly high number of pseudogenes related to keratin genes 8 and 18. J Cell Sci. 2001;114:2569–75.
Takahashi H, Shikata N, Tsubura A. Immunohistochemical reaction patterns of keratins in MNNG-induced shrew oesophageal carcinomas. Virchows Arch. 1994;424:267–71.
Takahashi H, Shikata N, Senzaki H, Shintaku M, Tsubura A. Immunohistochemical staining patterns of keratins in normal oesophageal epithelium and carcinoma of the oesophagus. Histopathology. 1995;26:45–50.
Chu PG, Lyda MH, Weiss LM. Cytokeratin 14 expression in epithelial neoplasms: a survey of 435 cases with emphasis on its value in differentiating squamous cell carcinomas from other epithelial tumours. Histopathology. 2001;39:9–16.
Yamazaki T, Saito K, Mori T, Date H, Arima S, Yoshida T, Date Y, Omatsu M, Sugiyama T, Suzuki K, Kunimura T, Morohoshi T. An immunohistological study on intraepithelial lesions in esophagus: expression of GLUT-1, cytokeratin and p53, with Ki-67 staining. Showa Univ J Med Sci. 2009;21:291–302.
Geddert H, Kiel S, Heep HJ, Gabbert HE, Sarbia M. The role of p63 and deltaNp63 (p40) protein expression and gene amplification in esophageal carcinogenesis. Hum Pathol. 2003;34:850–6.
Hu H, Xia SH, Li AD, Xu X, Cai Y, Han YL, Wei F, Chen BS, Huang XP, Han YS, Zhang JW, Zhang X, Wu M, Wang MR. Elevated expression of p63 protein in human esophageal squamous cell carcinomas. Int J Cancer. 2002;102:580–3.
Glickman JN, Yang A, Shahsafaei A, McKeon F, Odze RD. Expression of p53-related protein p63 in the gastrointestinal tract and in esophageal metaplastic and neoplastic disorders. Hum Pathol. 2001;32:1157–65.
Parsa R, Yang A, McKeon F, Green H. Association of p63 with proliferative potential in normal and neoplastic human keratinocytes. J Invest Dermatol. 1999;113:1099–105.
Mills AA, Zheng B, Wang XJ, Vogel H, Roop DR, Bradley A. p63 is a p53 homologue required for limb and epidermal morphogenesis. Nature. 1999;398:708–13.
Tanière P, Martel-Planche G, Saurin JC, Lombard-Bohas C, Berger F, Scoazec JY, Hainaut P. TP53 mutations, amplification of P63 and expression of cell cycle proteins in squamous cell carcinoma of the oesophagus from a low incidence area in Western Europe. Br J Cancer. 2001;85:721–6.
Suliman Y, Opitz OG, Avadhani A, Burns TC, El-Deiry W, Wong DT, Rustgi AK. p63 expression is associated with p53 loss in oral-esophageal epithelia of p53-deficient mice. Cancer Res. 2001;61:6467–73.
Parenti AR, Rugge M, Frizzera E, Ruol A, Noventa F, Ancona E, Ninfo V. p53 overexpression in the multistep process of esophageal carcinogenesis. Am J Surg Pathol. 1995;19:1418–22.
Ohbu M, Kobayashi N, Okayasu I. Expression of cell cycle regulatory proteins in the multistep process of oesophageal carcinogenesis: stepwise over-expression of cyclin E and p53, reduction of p21WAF1/CIP1 and dysregulation of cyclin D1 and p27KIP1. Histopathology. 2001;39:589–96.
Gao H, Wang LD, Zhou Q, Hong JY, Huang TY, Yang CS. p53 tumor suppressor gene mutation in early esophageal precancerous lesions and carcinoma among high-risk populations in Henan, China. Cancer Res. 1994;54:4342–6.
Kaneko K, Katagiri A, Konishi K, Kurahashi T, Ito H, Kumekawa Y, Yamamoto T, Muramoto T, Kubota Y, Nozawa H, Makino R, Kushima M, Imawari M. Study of p53 gene alteration as a biomarker to evaluate the malignant risk of Lugol-unstained lesion with non-dysplasia in the oesophagus. Br J Cancer. 2007;96:492–8.
Hinds PW, Finlay CA, Quartin RS, Baker SJ, Fearon ER, Vogelstein B, Levine AJ. Mutant p53 DNA clones from human colon carcinomas cooperate with ras in transforming primary rat cells: a comparison of the “hot spot” mutant phenotypes. Cell Growth Differ. 1990;1:571–80.
Wang WC, Wu TT, Chandan VS, Lohse CM, Zhang L. Ki-67 and ProExC are useful immunohistochemical markers in esophageal squamous intraepithelial neoplasia. Hum Pathol. 2011;42:1430–7.
Dixon MF. Gastrointestinal epithelial neoplasia: Vienna revisited. Gut. 2005;51:130–1.
Acknowledgments
We thank Mr. Hiroshi Abe, Department of Human Pathology, Juntendo University School of Medicine, for his expert technical assistance.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Nakayama, H., Mitomi, H., Imamhasan, A. et al. Stepwise overexpression of p63, p53, and cytokeratin 14 during progression of esophageal squamous intraepithelial neoplasia: useful immunohistochemical markers for differential diagnosis. Esophagus 9, 1–8 (2012). https://doi.org/10.1007/s10388-011-0302-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10388-011-0302-8