Abstract
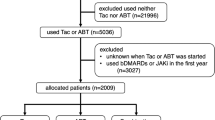
We assessed the safety of tacrolimus therapy for rheumatoid arthritis. Forty-two patients who started tacrolimus therapy between April 2005 and July 2006 were investigated retrospectively using data from their medical records up to June 2007. The cumulative treatment continuation rate was assessed by the Kaplan–Meier method. Fisher’s exact test was used to compare gastrointestinal symptoms between different tacrolimus doses and between the presence and absence of each concomitant medication. The mean (±SD) observation period was 288 ± 238 days. The cumulative treatment continuation rate was, respectively, 59.5% and 38.1% at 6 months and 1 year after the patients started treatment. Tacrolimus was discontinued in 28 patients, and was discontinued because of adverse reactions in 21 patients. Gastrointestinal symptoms were the most common adverse reactions (45.2% = 19/42 patients), followed by infections and hyperglycemia. Tacrolimus was discontinued in 9/19 patients with gastrointestinal symptoms, and was discontinued within 60 days of starting treatment in seven of them. Nausea and vomiting led to discontinuation in seven patients (within 60 days of starting treatment in six of them). The incidence of gastrointestinal symptoms was higher in patients receiving a daily dose ≥2 mg than in those receiving <2 mg/day. During treatment of rheumatoid arthritis by oral tacrolimus therapy, gastrointestinal symptoms were common, early, and dose-dependent. However, these symptoms were not severe and did not cause any serious safety problems.

Similar content being viewed by others
References
O’Dell JR (2005) Rheumatoid arthritis: the clinical picture. In: Koopman WJ, Moreland LW (eds) Arthritis and allied conditions: a textbook of rheumatology. vol. 1. 15th edn. Lippincott Williams & Wilkins, Philadelphia, pp 1165–1194
American College of Rheumatology Subcommittee on Rheumatoid Arthritis Guidelines (2002) Guidelines for the management of rheumatoid arthritis: 2002 update. Arthritis Rheum 46:328–346
Kino T, Hatanaka H, Miyata S et al (1987) FK-506, a novel immunosuppressant isolated from a Streptomyces. II. Immunosuppressive effect of FK-506 in vitro. J Antibiot 40:1256–1265
Kitahara K, Kawai S (2007) Cyclosporine and tacrolimus for the treatment of rheumatoid arthritis. Curr Opin Rheumatol 19:238–245
Shapiro R, Jordan ML, Scantlebury VP et al (1995) The superiority of tacrolimus in renal transplant recipients—the Pittsburgh experience. Clin Transpl 199–205
Testa G, Klintmalm GB (1997) Cyclosporine and tacrolimus: the mainstay of immunosuppressive therapy for solid organ transplantation. Clin Liver Dis 1:417–437
Gremillion RB, Posever JO, Manek N et al (1999) Tacrolimus (FK506) in the treatment of severe, refractory rheumatoid arthritis: initial experience in 12 patients. J Rheumatol 26:2332–2336
Kondo H, Abe T, Hashimoto H et al (2004) Efficacy and safety of tacrolimus (FK506) in treatment of rheumatoid arthritis: a randomized, double blind, placebo controlled dose-finding study. J Rheumatol 31:243–251
Kawai S, Hashimoto H, Kondo H et al (2006) Comparison of tacrolimus and mizoribine in a randomized, double-blind controlled study in patients with rheumatoid arthritis. J Rheumatol 33:2153–2161
Steinbrocker O, Traeger CH, Batterman RC (1949) Therapeutic criteria in rheumatoid arthritis. JAMA 140:659–662
Kawai S, Yamamoto K (2006) Safety of tacrolimus, an immunosuppressive agent, in the treatment of rheumatoid arthritis in elderly patients. Rheumatol (Oxf) 45:441–444
Yocum DE, Furst DE, Kaine JL et al (2003) Efficacy and safety of tacrolimus in patients with rheumatoid arthritis: a double-blind trial. Arthritis Rheum 48:3328–3337
Itoh Z, Suzuki T, Nakaya M et al (1984) Gastrointestinal motor-stimulating activity of macrolide antibiotics and analysis of their side effects on the canine gut. Antimicrob Agents Chemother 26:863–869
Itoh Z, Nakaya M, Suzuki T et al (1984) Erythromycin mimics exogenous motilin in gastrointestinal contractile activity in the dog. Am J Physiol 247:688–694
Itoh Z, Suzuki T, Nakaya M et al (1985) Structure–activity relation among macrolide antibiotics in initiation of interdigestive migrating contractions in the canine gastrointestinal tract. Am J Physiol 248:320–325
Costa A, Alessiani M, De Ponti F et al (1996) Stimulatory effect of FK506 and erythromycin on pig intestinal motility. Transplant Proc 28:2571–2572
Maes BD, Vanwalleghem J, Kuypers D et al (1999) Differences in gastric motor activity in renal transplant recipients treated with FK-506 versus cyclosporine. Transplantation 68:1482–1485
The U.S. Multicenter FK506 Liver Study Group (1994) A comparison of tacrolimus (FK506) and cyclosporine for immunosuppression in liver transplantation. N Engl J Med 331:1110–1115
Mayer AD, Dmitrewski J, Squifflet JP et al (1997) Multicenter randomized trial comparing tacrolimus (FK506) and cyclosporine in the prevention of renal allograft rejection: a report of the European Tacrolimus Multicenter Renal Study Group. Transplantation 64:436–443
Mor E, Schwersenz A, Sheiner PA et al (1994) Reversal of gastrointestinal toxicity associated with long-term FK506 immunosuppression by conversion to cyclosporine in liver transplant recipients. Transplantation 57:1130–1132
Astellas Pharma Inc. (2007) Prografâ (tacrolimus) prescribing information in Japan. Astellas Pharma, Tokyo
Fisher A, Schwartz M, Mor E et al (1994) Gastrointestinal toxicity associated with FK506 in liver transplant recipients. Transplant Proc 26:3106–3107
Ekberg H, Tedesco-Silva H, Demirbas A et al (2007) Reduced exposure to calcineurin inhibitors in renal transplantation. N Engl J Med 357:2562–2575
Pirsch JD, Miller J, Deierhoi MH et al (1997) A comparison of tacrolimus (FK506) and cyclosporine for immunosuppression after cadaveric renal transplantation. Transplantation 63:977–983
Acknowledgment
We wish to thank Ms. Sonoko Sakurai for her secretarial assistance.
Conflict of Interest Statement
Dr. Shinichi Kawai has served as a consultant to and/or received an honorarium from Astellas Pharma Inc. (Tokyo, Japan), the manufacturer of tacrolimus.
No conflict of interest has been declared by Kimiko Akimoto, Yoshie Kusunoki, Shinichiro Nishio, and Kenji Takagi.
Funding
This study was supported in part by a grant from The Japanese Ministry of Health, Labor and Welfare (S.K.).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Akimoto, K., Kusunoki, Y., Nishio, S. et al. Safety profile of tacrolimus in patients with rheumatoid arthritis. Clin Rheumatol 27, 1393–1397 (2008). https://doi.org/10.1007/s10067-008-0931-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10067-008-0931-z