Abstract
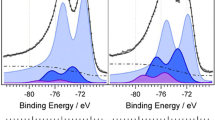
A prominent methanol-tolerant characteristic of the PtCeOx/C electrocatalyst was found during oxygen reduction reaction process. The carbon-supported platinum modified with cerium oxide (PtCeOx/C) as cathode electrocatalyst for direct methanol fuel cells was prepared via a simple and effective route. The synthesized electrocatalysts were characterized by X-ray diffraction and transmission electron microscopy. It was found that the cerium oxide within PtCeOx/C present in an amorphous form on the carbon support surface and the PtCeOx/C possesses almost similar disordered morphological structure and slightly smaller particle size compared with the unmodified Pt/C catalyst.





Similar content being viewed by others
References
Carrette L, Friedrich AK, Stimming U (2000) Chem Phys Chem 1:162. doi:10.1002/1439-7641(20001215)1:4<162::AID-CPHC162>3.0.CO;2-Z
McNicol BD, Rand DAJ, Williams KR (1999) J Power Sources 83:15. doi:10.1016/S0378-7753(99)00244-X
Raghuveer V, Ferreira PJ, Manthiram A (2006) Electrochem Commun 8:807. doi:10.1016/j.elecom.2006.03.022
Ren XM, Zelanay P, Thomas S, Davey J, Gottesfeld S (2000) J Power Sources 86:111. doi:10.1016/S0378-7753(99)00407-3
Kulikovsky AA, Schmitz H, Wippermann K, Mergel J, Fricke B, Sanders T, Sauer DU (2007) J Power Sources 173:420. doi:10.1016/j.jpowsour.2007.04.072
Aricò AS, Cretì P, Modica E, Monforte G, Baglio V, Antonucci V (2000) Electrochim Acta 45:4319. doi:10.1016/S0013-4686(00)00531-4
Jusys Z, Behm RJ (2004) Electrochim Acta 49:3891. doi:10.1016/j.electacta.2004.01.077
Urban PM, Funke A, Müller JT, Himmen M, Docter A (2001) Appl Catal Gen 221:459. doi:10.1016/S0926-860X(01)00819-5
Drillet J-F, Ee A, Friedemann J, Kötz R, Schnyder B, Schmidt VM (2002) Electrochim Acta 47:1983. doi:10.1016/S0013-4686(02)00027-0
Koffi RC, Coutanceau C, Garnier E, Léger J-M, Lamy C (2005) Electrochim Acta 50:4117. doi:10.1016/j.electacta.2005.01.028
Rajaram RR, Hayes JW, Ansell GP, Hatcher HA (1999) US Patent 5,993,762
Masui T, Hirai H, Hamada R, Imanaka N, Adachi G, Sakata T, Mori HJ (2003) Mater Chem 13:622. doi:10.1039/b208109a
Sahibzada M, Steele BCH, Zheng K, Rudkin RA, Metcalfe IS (1997) Catal Today 38:459. doi:10.1016/S0920-5861(97)00055-2
Yu HB, Kim J-H, Lee H-I, Scibioh MA, Lee J, Han J, Yoon SP, Ha HY (2005) J Power Sources 140:59. doi:10.1016/j.jpowsour.2004.08.015
Xu ZQ, Qi ZG, Kaufman A (2003) J Power Sources 115:40. doi:10.1016/S0378-7753(02)00721-8
Cant NW, Angove DE, Chambers DC (1998) Appl Catal B Environ 17:63
Kaspar J, Fornasiero P, Graziani M (1999) Catal Today 50:285. doi:10.1016/S0920-5861(98)00510-0
Shen PK, Xu CW (2006) Electrochem Commun 8:184. doi:10.1016/j.elecom.2005.11.013
Giordano N, Passalacqua E, Pino L, Aricò AS, Antonucci V, Vivaldi M, Kinoshita K (1991) Electrochim Acta 36:1979. doi:10.1016/0013-4686(91)85082-I
Aricò AS, Srinivasan S, Antonucci V (2001) Fuel Cells 1:133 doi:10.1002/1615-6854(200107)1:2<133::AID-FUCE133>3.0.CO;2-5
Klug HP, Alexander LE (1974) X-ray diffraction procedures for polycrystalline and amorphous materials. Wiley Interscience, New York, p 687
Yang H, Alonso-Vante N, Léger J-M, Lamy C (2004) J Phys Chem B 108:1938. doi:10.1021/jp030948q
Parsons R, Vandernoot T (1988) J Electroanal Chem 257:9. doi:10.1016/0022-0728(88)87028-1
Acknowledgments
The authors are grateful for the financial sponsors of State Key High Technology Research Program of China (863 Program, 2001AA323060 and 2003AA517062), Nature Science Foundation of China (20373068, 20433060) and Science and Technology Plan Project of Changchun (2004206).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Wang, X., Xu, W., Zhou, X. et al. PtCeOx/C as a novel methanol-tolerant electrocatalyst of oxygen reduction for direct methanol fuel cells. J Solid State Electrochem 13, 1449–1453 (2009). https://doi.org/10.1007/s10008-008-0720-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10008-008-0720-2