Abstract
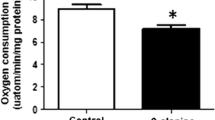
An important function of the β-amino acid, taurine, is the regulation of oxidative stress. However, taurine is neither a classical scavenger nor a regulator of the antioxidative defenses, leaving uncertain the mechanism underlying the antioxidant activity of taurine. In the present study, the taurine antagonist and taurine transport inhibitor, β-alanine, was used to examine the mechanism underlying the antioxidant activity of taurine. Exposure of isolated cardiomyocytes to medium containing β-alanine for a period of 48 h led to a 45% decrease in taurine content and an increase in mitochondrial oxidative stress, as evidenced by enhanced superoxide generation, the inactivation of the oxidant sensitive enzyme, aconitase, and the oxidation of glutathione. Associated with the increase in oxidative stress was a decline in electron transport activity, with the activities of respiratory chain complexes I and III declining 50–65% and oxygen consumption falling 30%. A reduction in respiratory chain activity coupled with an increase in oxidative stress is commonly caused by the development of a bottleneck in electron transport that leads to the diversion of electrons from the respiratory chain to the acceptor oxygen forming in the process superoxide. Because β-alanine exposure significantly reduces the levels of respiratory chain complex subunits, ND5 and ND6, the bottleneck in electron transport appears to be caused by impaired synthesis of key subunits of the electron transport chain complexes. Co-administration of taurine with β-alanine largely prevents the mitochondrial effects of β-alanine, but treatment of the cells with 5 mM taurine in the absence of β-alanine has no effect on the mitochondria, likely because taurine treatment has little effect on cellular taurine levels. Thus, taurine serves as a regulator of mitochondrial protein synthesis, thereby enhancing electron transport chain activity and protecting the mitochondria against excessive superoxide generation.






Similar content being viewed by others
References
Aruoma OI, Halliwell B, Hoey BM, Butler J (1988) The antioxidant action of taurine, hypotaurine and their metabolic precursors. Biochem J 256:251–255
Bouckenooghe T, Remacle C, Reusens B (2006) Is taurine a functional nutrient? Curr Opin Coln Nutr Metab Care 9:728–733
Chen Q, Vazquez EJ, Moghaddas S, Hoppel CL, Lesnefsky EJ (2003) Production of reactive oxygen species by mitochondria: central role of complex III. J Biol Chem 278:36927–36931
Eley DW, Lake N, ter Keurs HEDJ (1994) Taurine depletion and excitation-contraction coupling in rat myocardium. Circ Res 74:11210–11219
Gaull GE (1986) Taurine as a conditionally essential nutrient in man. J Am Coll Nutr 5:121–125
Grishko V, Pastukh V, Solodushko V, Gillespie M, Azuma J, Schaffer SW (2003) Apoptotic cascade initiated by angiotensin II in neonatal cardiomyocytes: role of DNA damage. Am J Physiol 285:H2364–H2372
Halestrap AP, Clarke SJ, Khalilin I (2007) The mitochondrial permeability transition pore—from molecular mechanism to reperfusion injury. In: Schaffer SW, Suleiman MS (eds) Mitochondria: the dynamic organelle. Springer Science + Business Media, New York, pp 241–269
Harada H, Cusack BJ, Olson RD, Stroo W, Azuma J, Hamaguchi T, Schaffer SW (1980) Taurine deficiency and doxorubicin: interaction with the cardiac sarcolemmal calcium pump. Biochem Pharm 39:745–751
Heller-Stilb B, van Roeyen C, Rascher K, Hartwig HG, Huth A, Seeliger MW, Warskulat U, Haeussinger D (2002) Disruption of the taurine transporter gene (taut) leads to retinal degeneration in mice. FASEB J 16:231–233
Huxtable RJ (1992) Physiological actions of taurine. Physiol Rev 72:101–163
Ito T, Kimura Y, Uozumi Y, Takai M, Muraoka S, Matsuda T, Ueki K, Yoshiyama M, Ikawa M, Okabe M, Schaffer SW, Fujio Y, Azuma J (2008) Taurine depletion caused by knocking out the taurine transporter gene leads to a cardiomyopathy with cardiac atrophy. J Mol Cell Cardiol 44:927–937
Jong CJ, Ito T, Mozaffari M, Azuma J, Schaffer S (2010) Effect of β-alanine treatment on mitochondrial taurine level and 5-taurinomethyluridine content. J Biomed Sci 17:S25
Kirino Y, Yasukawa T, Ohta S, Akira S, Ishihara K, Watanabe K, Suzuki T (2004) Codon-specific translational defect caused by wobble modification deficiency in mutant tRNA from a human mitochondrial disease. Proc Natl Acad Sci USA 101:15070–15075
Knopf K, Sturman JA, Armstrong M, Hayes KC (1978) Taurine: an essential nutrient for the cat. J Nutr 108:773–778
Pion PD, Kittleson MD, Rogers QR, Morris JG (1987) Myocardial failure in cats associated with low plasma taurine: a reversible cardiomyopathy. Science 237:764–768
Ricci C, Pastukh V, Leonard J, Turrens J, Wilson G, Schaffer D, Schaffer SW (2008) Mitochondrial DNA damage triggers mitochondrial-superoxide generation and apoptosis. Am J Physiol 294:C413–C422
Schaffer S, Solodushko V, Azuma J (2000) Taurine-deficient cardiomyopathy: role of phospholipids, calcium and osmotic stress. Adv Exp Med Biol 483:57–69
Schaffer SW, Solodushko V, Kakhniashvili D (2002) Beneficial effect of taurine depletion on osmotic sodium and calcium loading during chemical hypoxia. Am J Physiol 282:C1113–C1120
Schaffer S, Azuma J, Takahashi K, Mozaffari M (2003a) Why is taurine cytoprotective? In: Lombardini JB, Schaffer SW, Azuma J (eds) Taurine 5. Kluwer Academic/Plenum Publishers, New York, pp 307–321
Schaffer S, Solodushko V, Pastukh V, Ricci C, Azuma J (2003b) Possible cause of taurine deficient cardiomyopathy: potentiation of angiotensin II action. J Cardiovasc Pharmacol 41:751–759
Schaffer SW, Jong CJ, Ramila KC, Azuma J (2010) Physiological roles of taurine in heart and muscle. J Biomed Sci (in press)
Sturman JA (1993) Taurine in development. Physiol Rev 73:119–147
Turrens JF (2007) Formation of reactive oxygen species in mitochondria. In: Schaffer SW, Suleiman M-S (eds) Mitochondria: the dynamic organelle. Springer Science + Business Media, New York, pp 185–196
Wu JY, Prentice H (2010) Role of taurine in the central nervous system. J Biomed Sci 17(Suppl 1):S1
Acknowledgments
The study was supported with a grant from the American Heart Association.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Jong, C.J., Azuma, J. & Schaffer, S. Mechanism underlying the antioxidant activity of taurine: prevention of mitochondrial oxidant production. Amino Acids 42, 2223–2232 (2012). https://doi.org/10.1007/s00726-011-0962-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00726-011-0962-7