Abstract
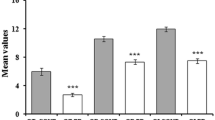
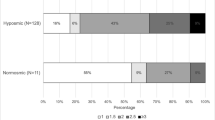
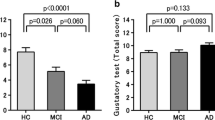
Mild cognitive impairment (MCI) and chemosensory dysfunction are non-motor symptoms of Parkinson’s disease (PD), but their association is unclear. We explored if MCI and the involvement of single cognitive domains influence olfaction and taste in PD. The role of demographic, clinical and neuropsychiatric variables was tested. We recruited 50 PD patients without dementia, no other reasons for cognitive impairment, no condition that could influence evaluation of cognition, olfaction and taste. They underwent a full neuropsychological and chemosensory (i.e., olfaction and taste) test with the Sniffin’ Sticks Extended test (SSET), Whole Mouth test (WMT) and Taste Strips test (TST). Fifty age- and sex-matched healthy subjects served as controls. Olfactory function and sweet identification were worse in PD than controls. MCI negatively influenced odor identification. Factors associated with poor olfactory function were age, overall cognition, apathy, and visuospatial dysfunction. Sour identification was affected by MCI and executive dysfunction, and salty identification by executive dysfunction. MCI, age and executive dysfunction worsened TST score. Awareness of olfactory dysfunction was impaired in PD with MCI. Education positively influenced SSET and TST scores. Our data confirmed that olfaction is abnormal in PD, while taste was only slightly impaired. Olfaction was worse in PD patients with visuospatial dysfunction, while sour and salty identification was worse in those with MCI and executive dysfunction, suggesting different underlying anatomical abnormalities. Future studies should incorporate neuroimaging and cerebrospinal fluid data to confirm this hypothesis. SSET odor identification and TST sour identification could be explored as quick screening tests for PD-MCI.




Similar content being viewed by others
References
Alzahrani H, Antonini A, Venneri A (2016) Apathy in mild Parkinson’s disease: neuropsychological and neuroimaging evidence. J Parkinsons Dis 6:821–832. https://doi.org/10.3233/JPD-160809
Appollonio I, Leone M, Isella V, Piamarta F, Consoli T, Villa ML, Forapani E, Russo A, Nichelli P (2005) The Frontal Assessment Battery (FAB): normative values in an Italian population sample. Neurol Sci 26:108–116. https://doi.org/10.1007/s10072-005-0443-4
Baba T, Kikuchi A, Hirayama K, Nishio Y, Hosokai Y, Kanno S, Hasegawa T, Sugeno N, Konno M, Suzuki K, Takahashi S, Fukuda H, Aoki M, Itoyama Y, Mori E, Takeda A (2012) Severe olfactory dysfunction is a prodromal symptom of dementia associated with Parkinson’s disease: a 3 year longitudinal study. Brain 135:161–169. https://doi.org/10.1093/brain/awr321
Benton AL, Varney NR, Hamsher KS (1978) Visuospatial judgment. A clinical test. Arch Neurol 35:364–367. https://doi.org/10.1001/archneur.1978.00500300038006
Besser LM, Litvan I, Monsell SE, Mock C, Weintraub S, Zhou XH, Kukull W (2016) Mild cognitive impairment in Parkinson’s disease versus Alzheimer’s disease. Parkinsonism Relat Disord 27:54–60. https://doi.org/10.1016/j.parkreldis.2016.04.007
Bohnen NI, Müller ML (2013) In vivo neurochemical imaging of olfactory dysfunction in Parkinson’s disease. J Neural Transm 120:571–576. https://doi.org/10.1007/s00702-012-0956-y
Bohnen NI, Müller ML, Kotagal V, Koeppe RA, Kilbourn MA, Albin RL, Frey KA (2010) Olfactory dysfunction, central cholinergic integrity and cognitive impairment in Parkinson’s disease. Brain 133:1747–1754. https://doi.org/10.1093/brain/awq079
Braak H, Del TK (2009) Neuroanatomy and pathology of sporadic Parkinson’s disease. Adv Anat Embryol Cell Biol 201:1–119
Brugnolo A, De Carli F, Accardo J, Amore M, Bosia LE, Bruzzaniti C, Cappa SF, Cocito L, Colazzo G, Ferrara M, Ghio L, Magi E, Mancardi GL, Nobili F, Pardini M, Rissotto R, Serrati C, Girtler N (2016) An updated Italian normative dataset for the Stroop color word test (SCWT). Neurol Sci 37:365–372. https://doi.org/10.1007/s10072-015-2428-2
Capasso R, Miceli G (2001) Esame Neuropsicologico per l’Afasia (E.N.P.A.). Springer, Milan
Carlesimo GA, Caltagirone G, Gainotti G, Fadda L, Gallassi R, Lorusso S, Marfia G, Marra C, Nocentini U, Parnetti L (1996) The mental deterioration battery: normative data, diagnostic reliability and qualitative analyses of cognitive impairment. Eur Neurol 36:378–384. https://doi.org/10.1159/000117297
Cecchini MP, Osculati F, Ottaviani S, Boschi F, Fasano A, Tinazzi M (2014) Taste performance in Parkinson’s disease. J Neural Transm (Vienna) 121:119–122. https://doi.org/10.1007/s00702-013-1089-7
Cecchini MP, Fasano A, Boschi F, Osculati F, Tinazzi M (2015) Taste in Parkinson’s disease. J Neurol 262:806–813. https://doi.org/10.1007/s00415-014-7518-1
Cecchini MP, Viviani D, Sandri M, Hähner A, Hummel T, Zancanaro C (2016) Olfaction in people with down syndrome: a comprehensive assessment across four decades of age. PLoS One 11:e0146486. https://doi.org/10.1371/journal.pone.0146486
Cecchini MP, Cardobi N, Sbarbati A, Monaco S, Tinazzi M, Tamburin S (2018) Post-traumatic taste disorders: a case series. J Neurol 265:836–844. https://doi.org/10.1007/s00415-018-8776-0
Cossu G, Melis M, Sarchioto M, Melis M, Melis M, Morelli M, Tomassini Barbarossa I (2018) 6-n-propylthiouracil taste disruption and TAS2R38 nontasting form in Parkinson’s disease. Mov Disord 33:1331–1339. https://doi.org/10.1002/mds.27391
Cramer CK, Friedman JH, Amick MM (2010) Olfaction and apathy in Parkinson’s disease. Parkinsonism Relat Disord 16:124–126. https://doi.org/10.1016/j.parkreldis.2009.09.004
Damholdt MF, Borghammer P, Larsen L, Ostergaard K (2011) Odor identification deficits identify Parkinson’s disease patients with poor cognitive performance. Mov Disord 26:2045–2050. https://doi.org/10.1002/mds.23782
de Bondt CC, Gerrits NJ, Veltman DJ, Berendse HW, van den Heuvel OA, van der Werf YD (2016) Reduced task-related functional connectivity during a set-shifting task in unmedicated early-stage Parkinson’s disease patients. BMC Neurosci 17:20. https://doi.org/10.1186/s12868-016-0254-y
Deeb J, Shah M, Muhammed N, Gunasekera R, Gannon K, Findley LJ, Hawkes CH (2010) A basic smell test is a sensitive as dopamine transporter scan: comparison of olfaction, taste and DaTSCAN in the diagnosis of Parkinson’s disease. QJM 103:941–952. https://doi.org/10.1093/qjmed/hcq142
Della Sala S, Laiacona M, Spinnler H, Ubezio C (1992) A cancellation test: its reliability in assessing attentional deficits in Alzheimer’s disease. Psychol Med 22:885–901. https://doi.org/10.1017/S0033291700038460
Djordjevic J, Jones-Gotman M, De Sousa K, Chertkow H (2008) Olfaction in patients with mild cognitive impairment and Alzheimer’s disease. Neurobiol Aging 29:693–706. https://doi.org/10.1016/j.neurobiolaging.2006.11.014
Domellöf ME, Lundin KF, Edström M, Forsgren L (2017) Olfactory dysfunction and dementia in newly diagnosed patients with Parkinson’s disease. Parkinsonism Relat Disord 38:41–47. https://doi.org/10.1016/j.parkreldis.2017.02.017
Doty RL, Deems DA, Stellar S (1988) Olfactory dysfunction in parkinsonism: a general deficit unrelated to neurologic signs, disease stage, or disease duration. Neurology 38:1237–1244
Doty RL, Nsoesie MT, Chung I, OsmanIan A, Pawasarat I, Caulfield J, Hurtig H, Silas J, Dubroff J, Duda JE, Ying GS, Tekeli H, Leon-Sarmiento FE (2015) Taste function in early stage treated and untreated Parkinson’s disease. J Neurol 262:547–557. https://doi.org/10.1007/s00415-014-7589-z
Doty RL, Chen JH, Overend J (2017) Taste quality confusions: influences of age, smoking, PTC taster status, and other subject characteristics. Perception 46:257–267. https://doi.org/10.1177/0301006616685577
Fastenau PS, Denburg NL, Mauer BA (1998) Parallel short forms for the Boston Naming test: psychometric properties and norms for older adults. J Clin Exp Neuropsychol 20:828–834
Federico A, Maier A, Vianello G, Mapelli D, Trentin M, Zanette G, Picelli A, Gandolfi M, Tamburin S (2015) Screening for mild cognitive impairment in Parkinson’s disease: comparison of the Italian versions of three neuropsychological tests. Parkinsons Dis 2015:681976. https://doi.org/10.1155/2015/681976
Federico A, Trentin M, Zanette G, Mapelli D, Picelli A, Smania N, Tinazzi M, Tamburin S (2017) Diagnosing mild cognitive impairment in Parkinson’s disease: which tests perform best in the Italian population?. Neurol Sci 38:1461–1468. https://doi.org/10.1007/s10072-017-3000-z
Fullard ME, Tran B, Xie SX, Toledo JB, Scordia C, Linder C, Purri R, Weintraub D, Duda JE, Chahine LM, Morley JF (2016) Olfactory impairment predicts cognitive decline in early Parkinson’s disease. Parkinsonism Relat Disord 25:45–51. https://doi.org/10.1016/j.parkreldis.2016.02.013
Garcia-Esparcia P, Schlüter A, Carmona M, Moreno J, Ansoleaga B, Torrejón-Escribano B, Gustincich S, Pujol A, Ferrer I (2013) Functional genomics reveals dysregulation of cortical olfactory receptors in Parkinson disease: novel putative chemoreceptors in the human brain. J Neuropathol Exp Neurol 72:524–539. https://doi.org/10.1097/NEN.0b013e318294fd76
Gjerde KV, Müller B, Skeie GO, Assmus J, Alves G, Tysnes OB (2018) Hyposmia in a simple smell test is associated with accelerated cognitive decline in early Parkinson’s disease. Acta Neurol Scand 138:508–514. https://doi.org/10.1111/ane.13003
Goldman JG, Holden S, Ouyang B, Bernard B, Goetz CG, Stebbins GT (2015) Diagnosing PD-MCI by MDS task force criteria: how many and which neuropsychological tests? Mov Disord 30:402–406. https://doi.org/10.1002/mds.26084
Good KP, Tourbier IA, Moberg P, Cuzzocreo JL, Geckle RJ, Yousem DM, Pham DL, Doty RL (2017) Unilateral olfactory sensitivity in multiple sclerosis. Physiol Behav 168:24–30. https://doi.org/10.1016/j.physbeh.2016.10.017
Haugen J, Müller MLTM, Kotagal V, Albin RL, Koeppe RA, Scott PJH, Frey KA, Bohnen NI (2016) Prevalence of impaired odor identification in Parkinson disease with imaging evidence of nigrostriatal denervation. J Neural Transm 123:421–424. https://doi.org/10.1007/s00702-016-1524-7
Hedner M, Larsson M, Arnold N, Zucco GM, Hummel T (2010) Cognitive factors in odor detection, odor discrimination, and odor identification tasks. J Clin Exp Neuropsychol 32:1062–1067. https://doi.org/10.1080/13803391003683070
Herman T, Weiss A, Brozgol M, Wilf-Yarkoni A, Giladi N, Hausdorff JM (2015) Cognitive function and other non-motor features in non-demented Parkinson’s disease motor subtypes. J Neural Transm 122:1115–1124. https://doi.org/10.1007/s00702-014-1349-1
Hong JY, Sunwoo MK, Ham JH, Lee JJ, Lee PH, Sohn YH (2015) Apathy and olfactory dysfunction in early Parkinson’s disease. J Mov Disord 8:21–25. https://doi.org/10.14802/jmd.14029
Hummel T, Kobal G, Gudziol H, Mackay – Sim A (2007) Normative data for the “Sniffin’ Sticks” including tests of odor identification, odor discrimination, and olfactory thresholds: an upgrade based on a group of more than 3000 subjects. Eur Arch Otorhinolaryngol 264:237–243. https://doi.org/10.1007/s00405-006-0173-0
Iannilli E, Stephan L, Hummel T, Reichmann H, Haehner A (2017) Olfactory impairment in Parkinson’s disease is a consequence of central nervous system decline. J Neurol 264:1236–1246. https://doi.org/10.1007/s00415-017-8521-0
Irwin DJ, Lee VM, Trojanowski JQ (2013) Parkinson’s disease dementia: convergence of alpha-synuclein, tau and amyloid-beta pathologies. Nat Rev Neurosci 14:626–636. https://doi.org/10.1038/nrn3549
Jellinger KA (2013) Mild cognitive impairment in Parkinson disease: heterogenous mechanisms. J Neural Transm 120:157–167. https://doi.org/10.1007/s00702-012-0771-5
Jellinger KA (2015) Neuropathobiology of non-motor symptoms in Parkinson disease. J Neural Transm 122:1429–1440. https://doi.org/10.1007/s00702-015-1405-5
Jellinger KA (2018) Dementia with Lewy bodies and Parkinson’s disease-dementia: current concepts and controversies. J Neural Transm 125:615–650. https://doi.org/10.1007/s00702-017-1821-9
Jung HJ, Shin IS, Lee JE (2019) Olfactory function in mild cognitive impairment and Alzheimer’s disease: a meta-analysis. Laryngoscope 129:362–369. https://doi.org/10.1002/lary.27399
Kawasaki I, Baba T, Takeda A, Mori E (2016) Loss of awareness of hyposmia is associated with mild cognitive impairment in Parkinson’s disease. Parkinsonism Relat Disord 22:74–79. https://doi.org/10.1016/j.parkreldis.2015.11.015
Kim HJ, Jeon BS, Lee JY, Cho YJ, Hong KS, Cho JY (2011) Taste function in patients with Parkinson’s disease. J Neurol 258:1076–1079. https://doi.org/10.1007/s00415-010-5884-x
Kim MS, Yoon JH, Kim HJ, Yong SW, Hong JM (2016) Olfactory dysfunction is related to postoperative delirium in Parkinson’s disease. J Neural Transm 123:589–594. https://doi.org/10.1007/s00702-016-1555-0
Kobayashi C, Kennedy LM (2002) Experience-induced changes in taste identification of monosodium glutamate. Physiol Behav 75:57–63. https://doi.org/10.1016/S0031-9384(01)00634-5
Landis BN, Welge-Luessen A, Brämerson A, Bende M, Mueller CA, Nordin S, Hummel T (2009) ‘‘Taste strips’’ a rapid, lateralized, gustatory bedside identification test based on impregnated filter papers. J Neurol 256:242–248. https://doi.org/10.1007/s00415-009-0088-y
Lang CJ, Leuschner T, Ulrich K, Stössel C, Heckmann JG, Hummel T (2006) Taste in dementing diseases and parkinsonism. J Neurol Sci 248:177–184
Litvan I, Goldman JG, Tröster AI, Schmand BA, Weintraub D, Petersen RC et al (2012) Diagnostic criteria for mild cognitive impairment in Parkinson’s disease: Movement Disorder Society task force guidelines. Mov Disord 27:349–356. https://doi.org/10.1002/mds.24893
Lötsch J, Reichmann H, Hummel T (2008) Different odor tests contribute differently to the evaluation of olfactory loss. Chem Sens 33:17–21
Marin RS, Biedrzycki RC, Firinciogullari S (1991) Reliability and validity of the Apathy Evaluation Scale. Psychiatry Res 38:143–162. https://doi.org/10.1016/0165-1781(91)90040-V
Masala C, Solla P, Liscia A, Defazio G, Saba L, Cannas A, Cavazzana A, Hummel T, Haehner A (2018) Correlation among olfactory function, motors’ symptoms, cognitive impairment, apathy, and fatigue in patients with Parkinson’s disease. J Neurol 265:1764–1771. https://doi.org/10.1007/s00415-018-8913-9
Mesholam RI, Moberg PJ, Mahr RN, Doty RL (1998) Olfaction in neurodegenerative disease: a meta-analysis of olfactory functioning in Alzheimer’s and Parkinson’s diseases. Arch Neurol 55:84–90
Mihaescu AS, Masellis M, Graff-Guerrero A, Kim J, Criaud M, Cho SS, Ghadery C, Valli M, Strafella AP (2018) Brain degeneration in Parkinson’s disease patients with cognitive decline: a coordinate-based meta-analysis. Brain Imaging Behav. https://doi.org/10.1007/s11682-018-9922-0
Moberg PJ, Balderston CC, Rick JH, Roalf DR, Weintraub D, Kleiner-Fisman G, Stern MB, Duda JE (2007) Phenylthiocarbamide (PTC) perception in Parkinson disease. Cogn Behav Neurol 20:145–148
Monastero R, Di Fiore P, Ventimiglia GD, Camarda R, Camarda C (2013) The neuropsychiatric profile of Parkinson’s disease subjects with and without mild cognitive impairment. J Neural Transm 120:607–611. https://doi.org/10.1007/s00702-013-0988-y
Mondini S, Mapelli D, Vestri A, Arcara G, Bisiacchi PS (2011) Esame neuropsicologico Breve 2, ENB-2. Raffaello Cortina Editore, Milan
Morley JF, Weintraub D, Mamikonyan E, Moberg PJ, Siderowf AD, Duda JE (2011) Olfactory dysfunction is associated with neuropsychiatric manifestations in Parkinson’s disease. Mov Disord 26:2051–2057. https://doi.org/10.1002/mds.23792
Mueller C, Kallert S, Renner B, Stiassny K, Temmel AF, Hummel T, Kobal G (2003) Quantitative assessment of gustatory function in a clinical context using impregnated ‘‘taste strips’’. Rhinology 41:2–6
Orhan KS, Karabulut B, Keleş N, Değer K (2012) Evaluation of factors concerning the olfaction using the Sniffin’ Sticks test. Otolaryngol Head Neck Surg 146:240–246. https://doi.org/10.1177/0194599811425019
Ottaviano G, Frasson G, Nardello E, Martini A (2016) Olfaction deterioration in cognitive disorders in the elderly. Aging Clin Exp Res 28:37–45. https://doi.org/10.1007/s40520-015-0380-x
Park JW, Kwon DY, Choi JH, Park MH, Yoon HK (2018) Olfactory dysfunctions in drug-naïve Parkinson’s disease with mild cognitive impairment. Parkinsonism Relat Disord 46:69–73. https://doi.org/10.1016/j.parkreldis.2017.11.334
Rahayel S, Frasnelli J, Joubert S (2012) The effect of Alzheimer’s disease and Parkinson’s disease on olfaction: a meta-analysis. Behav Brain Res 231:60–74. https://doi.org/10.1016/j.bbr.2012.02.047
Ricatti MJ, Ottaviani S, Boschi F, Fasano A, Tinazzi M, Cecchini MP (2017) A prospective evaluation of taste in Parkinson’s disease. J Neural Transm (Vienna) 124:347–352. https://doi.org/10.1007/s00702-016-1638-y
Risso DS, Mezzavilla M, Pagani L, Robino A, Morini G, Tofanelli S, Carrai M, Campa D, Barale R, Caradonna F, Gasparini P, Luiselli D, Wooding S, Drayna D (2016) Global diversity in the TAS2R38 bitter taste receptor: revisiting a classic evolutionary PROPosal. Sci Rep 6:25506. https://doi.org/10.1038/srep25506
Roalf DR, Moberg MJ, Turetsky BI, Brennan L, Kabadi S, Wolk DA, Moberg PJ (2017) A quantitative meta-analysis of olfactory dysfunction in mild cognitive impairment. J Neurol Neurosurg Psychiatry 88:226–232. https://doi.org/10.1136/jnnp-2016-314638
Shah M, Deeb J, Fernando M, Noyce A, Visentin E, Findley LJ, Hawkes CH (2009) Abnormality of taste and smell in Parkinson’s disease. Parkinsonism Relat Disord 15:232–237. https://doi.org/10.1016/j.parkreldis.2008.05.008
Sienkiewicz-Jarosz H, Scinska A, Kuran W, Ryglewicz D, Rogowski A, Wrobel E, Korkosz A, Kukwa A, Kostowski W, Bienkowski P (2005) Taste responses in patients with Parkinson’s disease. J Neurol Neurosurg Psychiatry 76:40–46. https://doi.org/10.1136/jnnp.2003.033373
Sienkiewicz-Jarosz H, Scinska A, Swiecicki L, Lipczynska-Lojkowska W, Kuran W, Ryglewicz D, Kolaczkowski M, Samochowiec J, Bienkowski P (2013) Sweet liking in patients with Parkinson’s disease. J Neurol Sci 329:17–22. https://doi.org/10.1016/j.jns.2013.03.005
Tomlinson CL, Stowe R, Patel S, Rick C, Gray R, Clarke CE (2010) Systematic review of levodopa dose equivalency reporting in Parkinson’s disease. Mov Disord 25:2649–2653. https://doi.org/10.1002/mds.23429
Weintraub D, Simuni T, Caspell-Garcia C, Coffey C, Lasch S, Siderowf A et al (2015) Cognitive performance and neuropsychiatric symptoms in early, untreated Parkinson’s disease. Mov Disord 30:919–927. https://doi.org/10.1002/mds.26170
White TL, Sadikot AF, Djordjevic J (2016) Metacognitive knowledge of olfactory dysfunction in Parkinson’s disease. Brain Cogn 104:1–6. https://doi.org/10.1016/j.bandc.2016.01.004
Wills AA, Elm JJ, Ye R, Chou KL, Parashos SA, Hauser RA, Bodis-Wollner I, Hinson VK, Christine CW, Schneider JS, The NINDS NET-PD Investigators (2016) Cognitive function in 1736 participants in NINDS exploratory trials in PD long-term study-1. Parkinsonism Relat Disord 33:127–133. https://doi.org/10.1016/j.parkreldis.2016.10.005
Wu L, Liu FT, Ge JJ, Zhao J, Tang YL, Yu WB, Yu H, Anderson T, Zuo CT, Chen L, Wang J (2018) Clinical characteristics of cognitive impairment in patients with Parkinson’s disease and its related pattern in 18 F-FDG PET imaging. Hum Brain Mapp. https://doi.org/10.1002/hbm.24311
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Author information
Authors and Affiliations
Contributions
MPC, AF and ST designed the study. MPC, AF, AZ, EM, CM, MT and ST collected the data. MPC, AF and ST analysed the data and made the statistical analysis. All Authors contributed to the interpretation of the data. MPC, AF and ST drafted the original version of the manuscript, which was revised critically by AZ, EM, CM and MT. All Authors approved the final version of the manuscript to be published.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Cecchini, M.P., Federico, A., Zanini, A. et al. Olfaction and taste in Parkinson’s disease: the association with mild cognitive impairment and the single cognitive domain dysfunction. J Neural Transm 126, 585–595 (2019). https://doi.org/10.1007/s00702-019-01996-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00702-019-01996-z