Abstract
Purpose
This study aimed at evaluating our hypothesis that tumour cells, which pass through the intraoperative cell salvage (IOCS) machine, lose viability due to possible injury to the cell membrane during centrifugation and filtration, enabling safe reinfusion even without filtration.
Methods
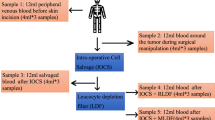
Thirteen patients who underwent metastatic spine tumour surgery (MSTS) at our institution were recruited. Blood samples (5 ml each) were collected at five different stages during surgery, namely, stage A and B: from patients’ vein during induction and at the time of maximum tumour manipulation; stage C, D and E: from the operative blood prior to IOCS processing, after IOCS processing and after IOCS-LDF (leucocyte depletion filter) processing, respectively. The samples were then analysed for viability of tumour cells using microwell-based culture.
Results
The median age of the patients was 65 years (range 37–77 years). The most common primary tumour was lung, followed by breast, hepatocellular and renal cell carcinoma. The median blood loss was 680 ml (range 300–1500 ml). Analysis of cultured blood samples showed that CTC-containing clusters were developed from some samples before IOCS-LDF processing (stage A: three patients, stage B: three patients and stage C: one patient). None of the samples from stages D and E generated clusters after culture, suggesting the absence of viable cancer cells after IOCS processing.
Conclusions
The salvaged blood may contain some tumour cells after processing with IOCS machine, but these cells are damaged and hence unable to replicate and unlikely to metastasise. The results of this study support the hypothesis that salvaged blood in MSTS is safe for transfusion.



Similar content being viewed by others
References
Witham TF, Khavkin YA, Gallia GL, Wolinsky JP, Gokaslan ZL (2006) Surgery insight: current management of epidural spinal cord compression from metastatic spine disease. Nat Clin Pract Neurol 2:87–94. doi:10.1038/ncpneuro0116 (quiz 116)
Chen Y, Tai BC, Nayak D, Kumar N, Lim JW, Goy RWL, Wong HK (2013) Blood loss in spinal tumour surgery and surgery for metastatic spinal disease: a meta-analysis. Bone Joint J 95-B:683–688
Kawai A, Kadota H, Yamaguchi U, Morimoto Y, Ozaki T, Beppu Y (2005) Blood loss and transfusion associated with musculoskeletal tumor surgery. J Surg Oncol 92:52–58. doi:10.1002/jso.20375
Blajchman MA, Bordin JO (1995) The tumor growth-promoting effect of allogeneic blood transfusions. Immunol Invest 24:311–317
Blumberg N (1997) Allogeneic transfusion and infection: economic and clinical implications. Semin Hematol 34:34–40
Kumar N, Ahmed Q, Lee VK, Chen Y, Zaw AS, Goy R, Agrawal RV, Dhewar AN, Wong HK (2014) Can there be a place for intraoperative salvaged blood in spine tumor surgery? Ann Surg Oncol 21:2436–2443. doi:10.1245/s10434-014-3569-x
Kumar N, Ahmed Q, Lee VK, Zaw AS, Goy R, Wong HK (2015) Are we ready for the use of intraoperative salvaged blood in metastatic spine tumour surgery? Eur Spine J. doi:10.1007/s00586-015-4112-x
Bower MR, Ellis SF, Scoggins CR, McMasters KM, Martin RC (2011) Phase II comparison study of intraoperative autotransfusion for major oncologic procedures. Ann Surg Oncol 18:166–173. doi:10.1245/s10434-010-1228-4
Catling S, Williams S, Freites O, Rees M, Davies C, Hopkins L (2008) Use of a leucocyte filter to remove tumour cells from intra-operative cell salvage blood. Anaesthesia 63:1332–1338. doi:10.1111/j.1365-2044.2008.05637.x (ANA5637 [pii])
Connor JP, Morris PC, Alagoz T, Anderson B, Bottles K, Buller RE (1995) Intraoperative autologous blood collection and autotransfusion in the surgical management of early cancers of the uterine cervix. Obstet Gynecol 86:373–378. doi:10.1016/0029-7844(95)00183-R
Ford BS, Sharma S, Rezaishiraz H, Huben RS, Mohler JL (2008) Effect of perioperative blood transfusion on prostate cancer recurrence. Urol Oncol 26:364–367. doi:10.1016/j.urolonc.2007.06.004
Kim JM, Kim GS, Joh JW, Suh KS, Park JB, Ko JS, Kwon CH, Yi NJ, Gwak MS, Lee KW, Kim SJ, Lee SK (2012) Long-term results for living donor liver transplant recipients with hepatocellular carcinoma using intraoperative blood salvage with leukocyte depletion filter. Transpl Int 26:84–89. doi:10.1111/tri.12001
Kumar N, Chen Y, Zaw AS, Nayak D, Ahmed Q, Soong R, Wong HK (2014) Use of intraoperative cell-salvage for autologous blood transfusions in metastatic spine tumour surgery: a systematic review. Lancet Oncol 15:e33–e41. doi:10.1016/S1470-2045(13)70245-6
Kumar N, Lam R, Zaw AS, Malhotra R, Tan J, Tan G, Setiobudi T (2014) Flow cytometric evaluation of the safety of intraoperative salvaged blood filtered with leucocyte depletion filter in spine tumour surgery. Ann Surg Oncol 21:4330–4335. doi:10.1245/s10434-014-3950-9
Martin RC, Wellhausen SR, Moehle DA, Martin AW, McMasters KM (2005) Evaluation of intraoperative autotransfusion filtration for hepatectomy and pancreatectomy. Ann Surg Oncol 12:1017–1024. doi:10.1245/ASO.2005.12.018
Perseghin P, Vigano M, Rocco G, Della Pona C, Buscemi A, Rizzi A (1997) Effectiveness of leukocyte filters in reducing tumor cell contamination after intraoperative blood salvage in lung cancer patients. Vox Sang 72:221–224
Khoo BL, Lee SC, Kumar P, Tan TZ, Warkiani ME, Ow SG, Nandi S, Lim CT, Thiery JP (2015) Short-term expansion of breast circulating cancer cells predicts response to anti-cancer therapy. Oncotarget 6:15578–15593
Alix-Panabieres C, Pantel K (2013) Circulating tumor cells: liquid biopsy of cancer. Clin Chem 59:110–118. doi:10.1373/clinchem.2012.194258
Marrinucci D, Bethel K, Lazar D, Fisher J, Huynh E, Clark P, Bruce R, Nieva J, Kuhn P (2010) Cytomorphology of circulating colorectal tumor cells: a small case series. J Oncol 2010:861341. doi:10.1155/2010/861341
Yu M, Bardia A, Wittner BS, Stott SL, Smas ME, Ting DT, Isakoff SJ, Ciciliano JC, Wells MN, Shah AM, Concannon KF, Donaldson MC, Sequist LV, Brachtel E, Sgroi D, Baselga J, Ramaswamy S, Toner M, Haber DA, Maheswaran S (2013) Circulating breast tumor cells exhibit dynamic changes in epithelial and mesenchymal composition. Science 339:580–584. doi:10.1126/science.1228522
Lucci A, Hall CS, Lodhi AK, Bhattacharyya A, Anderson AE, Xiao L, Bedrosian I, Kuerer HM, Krishnamurthy S (2012) Circulating tumour cells in non-metastatic breast cancer: a prospective study. Lancet Oncol 13:688–695. doi:10.1016/S1470-2045(12)70209-7
Bidard FC, Mathiot C, Delaloge S, Brain E, Giachetti S, de Cremoux P, Marty M, Pierga JY (2010) Single circulating tumor cell detection and overall survival in nonmetastatic breast cancer. Ann Oncol: Off J Eur Soc Med Oncol/ESMO 21:729–733. doi:10.1093/annonc/mdp391
Pierga JY, Bidard FC, Mathiot C, Brain E, Delaloge S, Giachetti S, de Cremoux P, Salmon R, Vincent-Salomon A, Marty M (2008) Circulating tumor cell detection predicts early metastatic relapse after neoadjuvant chemotherapy in large operable and locally advanced breast cancer in a phase II randomized trial. Clin Cancer Res: Off J Am Assoc Cancer Res 14:7004–7010. doi:10.1158/1078-0432.CCR-08-0030
Poste G, Fidler IJ (1980) The pathogenesis of cancer metastasis. Nature 283:139–146
Paget S (1889) The distribution of secondary growths in cancer of the breast. Lancet 133:571–573
Allan AL, Keeney M (2010) Circulating tumor cell analysis: technical and statistical considerations for application to the clinic. J Oncol 2010:426218. doi:10.1155/2010/426218
Chambers AF, Groom AC, MacDonald IC (2002) Dissemination and growth of cancer cells in metastatic sites. Nat Rev Cancer 2:563–572. doi:10.1038/nrc865
Chambers AF, Naumov GN, Varghese HJ, Nadkarni KV, MacDonald IC, Groom AC (2001) Critical steps in hematogenous metastasis: an overview. Surg Oncol Clin North Am 10:243–255 (vii)
Pantel K, Brakenhoff RH (2004) Dissecting the metastatic cascade. Nat Rev Cancer 4:448–456. doi:10.1038/nrc1370
Woodhouse EC, Chuaqui RF, Liotta LA (1997) General mechanisms of metastasis. Cancer 80:1529–1537
Karczewski DM, Lema MJ, Glaves D (1994) The efficiency of an autotransfusion system for tumor cell removal from blood salvaged during cancer surgery. Anesth Analg 78:1131–1135
Gorin MA, Eldefrawy A, Manoharan M, Soloway MS (2012) Oncologic outcomes following radical prostatectomy with intraoperative cell salvage. World J Urol 30:379–383. doi:10.1007/s00345-011-0746-4
Muscari F, Suc B, Vigouroux D, Duffas JP, Migueres I, Mathieu A, Lavayssiere L, Rostaing L, Fourtanier G (2005) Blood salvage autotransfusion during transplantation for hepatocarcinoma: does it increase the risk of neoplastic recurrence? Transpl Int 18:1236–1239. doi:10.1111/j.1432-2277.2005.00207.x
Nieder AM, Manoharan M, Yang Y, Soloway MS (2007) Intraoperative cell salvage during radical cystectomy does not affect long-term survival. Urology 69:881–884. doi:10.1016/j.urology.2007.01.060
Park SY, Choi GS, Park JS, Kim HJ, Ryuk JP, Choi WH (2012) Influence of surgical manipulation and surgical modality on the molecular detection of circulating tumor cells from colorectal cancer. J Korean Surg Soc 82:356–364. doi:10.4174/jkss.2012.82.6.356
Liang J, Shen J, Chua S, Fan Y, Zhai J, Feng B, Cai S, Li Z, Xue X (2015) Does intraoperative cell salvage system effectively decrease the need for allogeneic transfusions in scoliotic patients undergoing posterior spinal fusion? A prospective randomized study. Eur Spine J 24:270–275. doi:10.1007/s00586-014-3282-2
Acknowledgments
The authors thank AOSpine for granting AOSpine East Asia Research Award 2015 [AOSEA(R)2015-03]. 2. National University Health System for granting Bridging grant 2014 (NUHSRO/2014/013/Bridging/09) for conducting this study.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
None.
Rights and permissions
About this article
Cite this article
Kumar, N., Zaw, A.S., Khoo, B.L. et al. Intraoperative cell salvage in metastatic spine tumour surgery reduces potential for reinfusion of viable cancer cells. Eur Spine J 25, 4008–4015 (2016). https://doi.org/10.1007/s00586-016-4478-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00586-016-4478-4