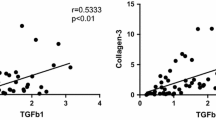
Abstract
Purpose
Chronic inflammation is thought to cause ligamentum flavum (LF) degeneration and hypertrophy in lumbar spinal canal stenosis (LSCS). Angiopoietin-like protein 2 (Angptl2) is highly expressed in hypertrophied LF. Because Angptl2 regulates interleukin-6 (IL-6) expression in various tissues, we investigated whether IL-6 is expressed in hypertrophied LF and, if so, does Angptl2 induce IL-6 expression in LF fibroblasts.
Methods
LF tissue was obtained from LSCS patients and non-LSCS patients. Polymerase chain reaction (PCR) for Angptl2 and IL-6 genes and immunohistochemistry for IL-6 protein were performed in LF tissue. Fibroblasts from LF tissue were used for in vitro experiments. Expression of integrin α5β1 (an Angptl2 receptor) and Angptl2 binding to receptors on LF fibroblasts were examined by fluorescence-activated cell sorter analysis and cell adhesion assays. After Angptl2 recombinant protein treatment, NF-κB activation and IL-6 expression in LF fibroblasts were investigated by immunocytochemistry, PCR, and enzyme-linked immunosorbent assay.
Results
IL-6 mRNA expression was increased in hypertrophied LF tissue from LSCS patients and positively correlated with LF thickness and Angptl2 mRNA expression. IL-6 protein was highly expressed in LF fibroblasts in hypertrophied LF tissue. In vitro experiments demonstrated integrin α5β1 expression on LF fibroblasts and Angptl2 binding to cells via receptors. Angptl2 stimulation promoted NF-κB nuclear translocation and induced IL-6 expression and secretion in LF fibroblasts.
Conclusions
Angptl2 promotes inflammation in LF tissue by activating IL-6 expression, leading to LF degeneration and hypertrophy.





Similar content being viewed by others
References
Sairyo K, Biyani A, Goel V, Leaman D, Booth R Jr, Thomas J, Gehling D, Vishnubhotla L, Long R, Ebraheim N (2005) Pathomechanism of ligamentum flavum hypertrophy: a multidisciplinary investigation based on clinical, biomechanical, histologic, and biologic assessments. Spine 30:2649–2656
Sairyo K, Biyani A, Goel VK, Leaman DW, Booth R Jr, Thomas J, Ebraheim NA, Cowgill IA, Mohan SE (2007) Lumbar ligamentum flavum hypertrophy is due to accumulation of inflammation-related scar tissue. Spine 32:E340–E347. doi:10.1097/01.brs.0000263407.25009.6e
Schräder P, Grob D, Rahn BA, Cordey J, Dvorak J (1999) Histology of the ligamentum flavum in patients with degenerative lumbar spinal stenosis. Eur Spine J 8:323–328
Moon HJ, Park YK, Ryu Y, Kim JH, Kwon TH, Chung HS (2012) The angiogenic capacity from ligamentum flavum subsequent to inflammation: a critical component of the pathomechanism of hypertrophy. Spine 37:E147–E155. doi:10.1097/BRS.0b013e3182269b19
Chen YT, Wei JD, Wang JP, Lee HH, Chiang ER, Lai HC, Chen LL, Lee YT, Tsai CC, Liu CL, Hung SC (2011) Isolation of mesenchymal stem cells from human ligamentum flavum: implicating etiology of ligamentum flavum hypertrophy. Spine 36:E1193–E1200. doi:10.1097/BRS.0b013e3182053f58
Park JO, Lee BH, Kang YM, Kim TH, Yoon JY, Kim H, Kwon UH, Lee KI, Lee HM, Moon SH (2013) Inflammatory cytokines induce fibrosis and ossification of human ligamentum flavum cells. J Spinal Disord Tech 26:E6–E12
Nakatani T, Marui T, Hitora T, Doita M, Nishida K, Kurosaka M (2002) Mechanical stretching force promotes collagen synthesis by cultured cells from human ligamentum flavum via transforming growth factor-beta1. J Orthop Res Off Publ Orthop Res Soc 20:1380–1386. doi:10.1016/s0736-0266(02)00046-3
Park JB, Chang H, Lee JK (2001) Quantitative analysis of transforming growth factor-beta 1 in ligamentum flavum of lumbar spinal stenosis and disc herniation. Spine 26:E492–E495
Border WA, Noble NA (1994) Transforming growth factor beta in tissue fibrosis. New Engl J Med 331:1286–1292. doi:10.1056/nejm199411103311907
Nakamura T, Okada T, Endo M, Kadomatsu T, Taniwaki T, Sei A, Odagiri H, Masuda T, Fujimoto T, Nakamura T, Oike Y, Mizuta H (2014) Angiopoietin-like protein 2 induced by mechanical stress accelerates degeneration and hypertrophy of the ligamentum flavum in lumbar spinal canal stenosis. PLoS One 9:e85542. doi:10.1371/journal.pone.0085542
Tabata M, Kadomatsu T, Fukuhara S, Miyata K, Ito Y, Endo M, Urano T, Zhu HJ, Tsukano H, Tazume H, Kaikita K, Miyashita K, Iwawaki T, Shimabukuro M, Sakaguchi K, Ito T, Nakagata N, Yamada T, Katagiri H, Kasuga M, Ando Y, Ogawa H, Mochizuki N, Itoh H, Suda T, Oike Y (2009) Angiopoietin-like protein 2 promotes chronic adipose tissue inflammation and obesity-related systemic insulin resistance. Cell Metab 10:178–188. doi:10.1016/j.cmet.2009.08.003
Okada T, Tsukano H, Endo M, Tabata M, Miyata K, Kadomatsu T, Miyashita K, Semba K, Nakamura E, Tsukano M, Mizuta H, Oike Y (2010) Synoviocyte-derived angiopoietin-like protein 2 contributes to synovial chronic inflammation in rheumatoid arthritis. Am J Pathol 176:2309–2319. doi:10.2353/ajpath.2010.090865
Tazume H, Miyata K, Tian Z, Endo M, Horiguchi H, Takahashi O, Horio E, Tsukano H, Kadomatsu T, Nakashima Y, Kunitomo R, Kaneko Y, Moriyama S, Sakaguchi H, Okamoto K, Hara M, Yoshinaga T, Yoshimura K, Aoki H, Araki K, Hao H, Kawasuji M, Oike Y (2012) Macrophage-derived angiopoietin-like protein 2 accelerates development of abdominal aortic aneurysm. Arterioscler Thromb Vasc Biol 32:1400–1409. doi:10.1161/ATVBAHA.112.247866
Aoi J, Endo M, Kadomatsu T, Miyata K, Nakano M, Horiguchi H, Ogata A, Odagiri H, Yano M, Araki K, Jinnin M, Ito T, Hirakawa S, Ihn H, Oike Y (2011) Angiopoietin-like protein 2 is an important facilitator of inflammatory carcinogenesis and metastasis. Cancer Res 71:7502–7512. doi:10.1158/0008-5472.can-11-1758
Endo M, Nakano M, Kadomatsu T, Fukuhara S, Kuroda H, Mikami S, Hato T, Aoi J, Horiguchi H, Miyata K, Odagiri H, Masuda T, Harada M, Horio H, Hishima T, Nomori H, Ito T, Yamamoto Y, Minami T, Okada S, Takahashi T, Mochizuki N, Iwase H, Oike Y (2012) Tumor cell-derived angiopoietin-like protein Angptl2 is a critical driver of metastasis. Cancer Res 72:1784–1794. doi:10.1158/0008-5472.can-11-3878
Ogata A, Endo M, Aoi J, Takahashi O, Kadomatsu T, Miyata K, Tian Z, Jinnin M, Fukushima S, Ihn H, Oike Y (2012) The role of angiopoietin-like protein 2 in pathogenesis of dermatomyositis. Biochem Biophys Res Commun 418:494–499. doi:10.1016/j.bbrc.2012.01.052
Odagiri H, Kadomatsu T, Endo M, Masuda T, Morioka MS, Fukuhara S, Miyamoto T, Kobayashi E, Miyata K, Aoi J, Horiguchi H, Nishimura N, Terada K, Yakushiji T, Manabe I, Mochizuki N, Mizuta H, Oike Y (2014) The secreted protein Angptl2 promotes metastasis of osteosarcoma cells through integrin alpha5beta1, p38 MAPK, and matrix metalloproteinases. Sci Signal 7:ra7. doi:10.1126/scisignal.2004612
Horio E, Kadomatsu T, Miyata K, Arai Y, Hosokawa K, Doi Y, Ninomiya T, Horiguchi H, Endo M, Tabata M, Tazume H, Tian Z, Takahashi O, Terada K, Takeya M, Hao H, Hirose N, Minami T, Suda T, Kiyohara Y, Ogawa H, Kaikita K, Oike Y (2014) Role of endothelial cell-derived Angptl2 in vascular inflammation leading to endothelial dysfunction and atherosclerosis progression. Arterioscler Thromb Vasc Biol. doi:10.1161/atvbaha.113.303116
Aoi J, Endo M, Kadomatsu T, Miyata K, Ogata A, Horiguchi H, Odagiri H, Masuda T, Fukushima S, Jinnin M, Hirakawa S, Sawa T, Akaike T, Ihn H, Oike Y (2014) Angiopoietin-like protein 2 accelerates carcinogenesis by activating chronic inflammation and oxidative stress. Mol Cancer Res MCR 12:239–249. doi:10.1158/1541-7786.mcr-13-0336
Gabay C (2006) Interleukin-6 and chronic inflammation. Arthritis Res Ther 8:3. doi:10.1186/ar1917
Knight DA, Ernst M, Anderson GP, Moodley YP, Mutsaers SE (2003) The role of gp130/IL-6 cytokines in the development of pulmonary fibrosis: critical determinants of disease susceptibility and progression? Pharmacol Ther 99:327–338
Mir SA, Chatterjee A, Mitra A, Pathak K, Mahata SK, Sarkar S (2012) Inhibition of signal transducer and activator of transcription 3 (STAT3) attenuates interleukin-6 (IL-6)-induced collagen synthesis and resultant hypertrophy in rat heart. J Biol Chem 287:2666–2677. doi:10.1186/ar1917
Camenisch G, Pisabarro MT, Sherman D, Kowalski J, Nagel M, Hass P, Xie MH, Gurney A, Bodary S, Liang XH, Clark K, Beresini M, Ferrara N, Gerber HP (2002) Angptl3 stimulates endothelial cell adhesion and migration via integrin alpha v beta 3 and induces blood vessel formation in vivo. J Biol Chem 277:17281–17290. doi:10.1074/jbc.M109768200
Zheng J, Umikawa M, Cui C, Li J, Chen X, Zhang C, Hyunh H, Kang X, Silvany R, Wan X, Ye J, Canto AP, Chen SH, Wang HY, Ward ES, Zhang CC (2012) Inhibitory receptors bind ANGPTLs and support blood stem cells and leukaemia development. Nature 485:656–660. doi:10.1038/nature11095
Libermann TA, Baltimore D (1990) Activation of interleukin-6 gene expression through the NF-kappa B transcription factor. Mol Cell Biol 10:2327–2334
Kong MH, Morishita Y, He W, Miyazaki M, Zhang H, Wu G, Hymanson HJ, Wang JC (2009) Lumbar segmental mobility according to the grade of the disc, the facet joint, the muscle, and the ligament pathology by using kinetic magnetic resonance imaging. Spine 34:2537–2554
Kawaguchi Y, Hara M, Wright TM (1999) Endogenous IL-1alpha from systemic sclerosis fibroblasts induces IL-6 and PDGF-A. J Clin Invest 103:1253–1260
Hasegawa M, Sato S, Ihn H, Takehara K (1999) Enhanced production of interleukin-6 (IL-6), oncostatin M and soluble IL-6 receptor by cultured peripheral blood mononuclear cells from patients with systemic sclerosis. Rheumatology 38:612–617
Stamenkovic I (2003) Extracellular matrix remodelling: the role of matrix metalloproteinases. J Pathol 200:448–464
Acknowledgments
We thank Ms. M. Nakata for technical assistance and Edanz for providing editorial assistance.
Conflict of interest
None.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
586_2015_3835_MOESM1_ESM.tif
Supplementary material 1 (TIFF 1461 kb) S-Fig. 1 Angptl2 receptor expression in each LF fibroblast. Fluorescence-activated cell sorter analysis of LF fibroblasts for expression of integrin α5β1 (left) or leukocyte immunoglobulin-like receptor B2 (LILRB2) (right). Representative expression patterns obtained with anti-α5β1 and anti-LILRB2 antibodies (unfilled peaks) or isotype-matched immunoglobulin G (IgG) (shaded peaks) are shown. Analysis was conducted using Flow Jo software (Treestar, Inc., San Carlos, CA, USA)
586_2015_3835_MOESM2_ESM.tif
Supplementary material 2 (TIFF 1034 kb) S-Fig. 2 Quantification of the adhesion of LF fibroblasts to Angptl2. Quantification of the adhesion of LF fibroblasts (n 3) mediated via integrin α5β1. OD595 value of Angptl2-coating was set at 1. Data are expressed as the mean ± SEM. **p < 0.01 versus Angptl2 coating
586_2015_3835_MOESM3_ESM.tif
Supplementary material 3 (TIFF 6507 kb) S-Fig. 3 Immunocytochemistry for NF-κB p65 after Angptl2 administration in each cell. Note the nuclear translocation of p65 by Angptl2 and inhibition of the translocation by anti-integrin α5β1 antibody in LF fibroblasts. Nuclei were stained with 4′,6′-diamidino-2-phenylindole (DAPI). Scale bars represent 50 μm in each panel
586_2015_3835_MOESM4_ESM.tif
Supplementary material 4 (TIFF 806 kb) S-Fig. 4 IL-6 expression by each LF fibroblasts in response to Angptl2. IL-6 mRNA expression in response to Angptl2 with or without BAY11-7085 (n = 3). The value of the controls (no Angptl2 treatment) was set at 1. Data are expressed as the mean ± SEM. **p < 0.01 compared with controls. †p < 0.01 compared with Angptl2 treatment
586_2015_3835_MOESM5_ESM.tif
Supplementary material 5 (TIFF 755 kb) S-Fig. 5 IL-6 secretion by each LF fibroblasts in response to Angptl2. IL-6 secretion (B) in response to Angptl2 with or without BAY11-7085 (n = 3). The value of the controls (no Angptl2 treatment) was set at 1. Data are expressed as the mean ± SEM. **p < 0.01 compared with controls. †p < 0.01 compared with Angptl2 treatment
Rights and permissions
About this article
Cite this article
Nakamura, T., Okada, T., Endo, M. et al. Angiopoietin-like protein 2 promotes inflammatory conditions in the ligamentum flavum in the pathogenesis of lumbar spinal canal stenosis by activating interleukin-6 expression. Eur Spine J 24, 2001–2009 (2015). https://doi.org/10.1007/s00586-015-3835-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00586-015-3835-z