Abstract
Introduction
Different approaches for disc regeneration are currently under investigation. Beside gene therapy and tissue engineering techniques, the application of growth and differentiation factors own promising potential. Studies using reduced intervertebral disc models, such as cell or tissue fragment cultures, have limited validity and show controversial results depending on the employed experimental model. Therefore, the goal of the current study was to investigate the effect of BMP-2 and TGF-β3 on intervertebral disc degeneration using an in vitro full-organ disc/endplate culture system.
Materials and methods
Intervertebral rabbit disc explants were cultured in the presence of 1 μg/ml BMP-2 or TGF-β3 for 21 days in DMEM/F12 media. Nucleus and annulus were analyzed for gene expression of collagen type I and II (Col I/II), aggrecan, collagenases (MMP-1/MMP-13) with RT-qPCR, histological changes with bone and proteoglycan-specific staining (von Kossa, toluidine blue) and differences in cellularity (DNA) and proteoglycan content (alcian blue binding assay).
Results
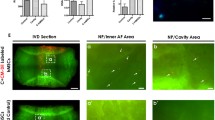
The results demonstrate that disc proteoglycan concentration decreased with time in the TGF-β3 and BMP-2 groups. In the annulus fibrosus (AF), TGF-β3 and BMP-2 resulted in an up-regulation of Col I and type II, and of aggrecan gene expression. In contrast, MMP genes were inhibited. In the nucleus, the growth factors decreased gene expression of aggrecan and spontaneous Col I up-regulation was inhibited by TGF-β3, whereas expression of Col II was decreased with BMP-2. There was no effect on expression of MMP-1 and MMP-13 for most sampling points. However, TGF-β3 and BMP-2 induced ossification of the AF was demonstrated by histology.
Conclusion
It can be concluded that both growth factors, at the tested concentrations, may not be suitable to regenerate the whole intervertebral disc organ but they are interesting candidates for being injected alone or in combination into a painful intervertebral disc to induce osseous fusion (spondylodesis).





Similar content being viewed by others
References
Aebi M, Arlet V, Webb J (eds) (2007) AOspine manual. Georg Thieme Verlag, Stuttgart and New York
McGirt MJ, Ambrossi GL, Datoo G, Sciubba DM, Witham TF, Wolinsky JP, Gokaslan ZL, Bydon A (2009) Recurrent disc herniation and long-term back pain after primary lumbar discectomy: review of outcomes reported for limited versus aggressive disc removal. Neurosurgery 64:338–344 (discussion 344–345)
Glassman SD, Carreon LY, Djurasovic M, Dimar JR, Johnson JR, Puno RM, Campbell MJ (2009) Lumbar fusion outcomes stratified by specific diagnostic indication. Spine J 9:13–21
Mirza SK, Deyo RA (2007) Systematic review of randomized trials comparing lumbar fusion surgery to nonoperative care for treatment of chronic back pain. Spine 32:816–823
Galbusera F, Bellini CM, Zweig T, Ferguson S, Raimondi MT, Lamartina C, Brayda-Bruno M, Fornari M (2008) Design concepts in lumbar total disc arthroplasty. Eur Spine J 17:1635–1650
Bothmann M, Kast E, Boldt GJ, Oberle J (2008) Dynesys fixation for lumbar spine degeneration. Neurosurg Rev 31:189–196
Freburger JK, Holmes GM, Agans RP, Jackman AM, Darter JD, Wallace AS, Castel LD, Kalsbeek WD, Carey TS (2009) The rising prevalence of chronic low back pain. Arch Intern Med 169:251–258
Wenig CM, Schmidt CO, Kohlmann T, Schweikert B (2009) Costs of back pain in Germany. Eur J Pain 13:280–286
Gandjour A, Telzerow A, Lauterbach KW (2005) European comparison of costs and quality in the treatment of acute back pain. Spine 30:969–975
Masuda K (2008) Biological repair of the degenerated intervertebral disc by the injection of growth factors. Eur Spine J 17(4):441–451
Suzuki T, Nishida K, Kakutani K, Maeno K, Yurube T, Takada T, Kurosaka M, Doita M (2009) Sustained long-term RNA interference in nucleus pulposus cells in vivo mediated by unmodified small interfering RNA. Eur Spine J 18:263–270
Sheikh H, Zakharian K, De La Torre RP, Facek C, Vasquez A, Chaudhry GR, Svinarich D, Perez-Cruet MJ (2009) In vivo intervertebral disc regeneration using stem cell-derived chondroprogenitors. J Neurosurg Spine 10:265–272
Richardson SM, Hoyland JA (2008) Stem cell regeneration of degenerated intervertebral discs: current status. Curr Pain Headache Rep 12:83–88
Le Maitre CL, Baird P, Freemont AJ, Hoyland JA (2009) An in vitro study investigating the survival and phenotype of mesenchymal stem cells following injection into nucleus pulposus tissue. Arthr Res Ther 11:R20
Hiyama A, Mochida J, Sakai D (2008) Stem cell applications in intervertebral disc repair. Cell Mol Biol (Noisy-le-grand) 54:24–32
Pratsinis H, Kletsas D (2008) Growth factors in intervertebral disc homeostasis. Connect Tissue Res 49:273–276
Buttle DJ (2007) Factors controlling matrix turnover in health and disease. Biochem Soc Trans 35:643–646
Masuda K, An HS (2006) Prevention of disc degeneration with growth factors. Eur Spine J 15(Suppl 3):S422–S432
Gordon KJ, Blobe GC (2008) Role of transforming growth factor-beta superfamily signaling pathways in human disease. Biochim Biophys Acta 1782:197–228
Blobe GC, Schiemann WP, Lodish HF (2000) Role of transforming growth factor beta in human disease. N Engl J Med 342:1350–1358
Herpin A, Lelong C, Favrel P (2004) Transforming growth factor-beta-related proteins: an ancestral and widespread superfamily of cytokines in metazoans. Dev Comp Immunol 28:461–485
Assoian RK, Komoriya A, Meyers CA, Miller DM, Sporn MB (1983) Transforming growth factor-beta in human platelets. Identification of a major storage site, purification, and characterization. J Biol Chem 258:7155–7160
Chen D, Zhao M, Mundy GR (2004) Bone morphogenetic proteins. Growth Factors 22:233–241
Wozney JM (1989) Bone morphogenetic proteins. Prog Growth Factor Res 1:267–280
Schimandle JH, Boden SD, Hutton WC (1995) Experimental spinal fusion with recombinant human bone morphogenetic protein-2. Spine 20:1326–1337
Hecht BP, Fischgrund JS, Herkowitz HN, Penman L, Toth JM, Shirkhoda A (1999) The use of recombinant human bone morphogenetic protein 2 (rhBMP-2) to promote spinal fusion in a nonhuman primate anterior interbody fusion model. Spine 24:629–636
Boden SD, Kang J, Sandhu H, Heller JG (2002) Use of recombinant human bone morphogenetic protein-2 to achieve posterolateral lumbar spine fusion in humans: a prospective, randomized clinical pilot trial: 2002 Volvo Award in clinical studies. Spine 27:2662–2673
Burkus JK, Sandhu HS, Gornet MF, Longley MC (2005) Use of rhBMP-2 in combination with structural cortical allografts: clinical and radiographic outcomes in anterior lumbar spinal surgery. J Bone Joint Surg Am 87:1205–1212
Meisel HJ, Schnoring M, Hohaus C, Minkus Y, Beier A, Ganey T, Mansmann U (2008) Posterior lumbar interbody fusion using rhBMP-2. Eur Spine J 17:1735–1744
Carlisle E, Fischgrund JS (2005) Bone morphogenetic proteins for spinal fusion. Spine J 5:240S–249S
Tim Yoon S, Su Kim K, Li J, Soo Park J, Akamaru T, Elmer WA, Hutton WC (2003) The effect of bone morphogenetic protein-2 on rat intervertebral disc cells in vitro. Spine 28:1773–1780
Zhang Y, Li Z, Thonar EJ, An HS, He TC, Pietryla D, Phillips FM (2005) Transduced bovine articular chondrocytes affect the metabolism of cocultured nucleus pulposus cells in vitro: implications for chondrocyte transplantation into the intervertebral disc. Spine 30:2601–2607
Huang KY, Yan JJ, Hsieh CC, Chang MS, Lin RM (2007) The in vivo biological effects of intradiscal recombinant human bone morphogenetic protein-2 on the injured intervertebral disc: an animal experiment. Spine 32:1174–1180
Kong MH, Do DH, Miyazaki M, Wei F, Yoon SH, Wang JC (2008) Rabbit model for in vivo study of intervertebral disc degeneration and regeneration. J Korean Neurosurg Soc 44:327–333
Miyamoto K, Masuda K, Kim JG, Inoue N, Akeda K, Andersson GB, An HS (2006) Intradiscal injections of osteogenic protein-1 restore the viscoelastic properties of degenerated intervertebral discs. Spine J 6:692–703. doi:10.1016/j.spinee.2006.04.014
Imai Y, Miyamoto K, An HS, Thonar EJ, Andersson GB, Masuda K (2007) Recombinant human osteogenic protein-1 upregulates proteoglycan metabolism of human anulus fibrosus and nucleus pulposus cells. Spine (Phila Pa 1976) 32:1303–1309. doi:10.1097/BRS.0b013e3180593238 (discussion 1310)
Matsunaga S, Nagano S, Onishi T, Morimoto N, Suzuki S, Komiya S (2003) Age-related changes in expression of transforming growth factor-beta and receptors in cells of intervertebral discs. J Neurosurg 98:63–67
Gruber HE, Fisher EC Jr, Desai B, Stasky AA, Hoelscher G, Hanley EN Jr (1997) Human intervertebral disc cells from the annulus: three-dimensional culture in agarose or alginate and responsiveness to TGF-beta1. Exp Cell Res 235:13–21
Nishida K, Kang JD, Gilbertson LG, Moon SH, Suh JK, Vogt MT, Robbins PD, Evans CH (1999) Modulation of the biologic activity of the rabbit intervertebral disc by gene therapy: an in vivo study of adenovirus-mediated transfer of the human transforming growth factor beta 1 encoding gene. Spine 24:2419–2425
Walsh AJ, Bradford DS, Lotz JC (2004) In vivo growth factor treatment of degenerated intervertebral discs. Spine 29:156–163
Steffen T, Stoll T, Arvinte T, Schenk RK (2001) Porous tricalcium phosphate and transforming growth factor used for anterior spine surgery. Eur Spine J 10(Suppl 2):S132–S140
Risbud MV, Di Martino A, Guttapalli A, Seghatoleslami R, Denaro V, Vaccaro AR, Albert TJ, Shapiro IM (2006) Toward an optimum system for intervertebral disc organ culture: TGF-beta 3 enhances nucleus pulposus and anulus fibrosus survival and function through modulation of TGF-beta-R expression and ERK signaling. Spine 31:884–890
Haschtmann D, Stoyanov JV, Ettinger L, Nolte LP, Ferguson SJ (2006) Establishment of a novel intervertebral disc/endplate culture model: analysis of an ex vivo in vitro whole-organ rabbit culture system. Spine 31:2918–2925
Haschtmann D, Stoyanov JV, Ferguson SJ (2006) Influence of diurnal hyperosmotic loading on the metabolism and matrix gene expression of a whole-organ intervertebral disc model. J Orthop Res 24:1957–1966
Haschtmann D, Stoyanov JV, Gedet P, Ferguson SJ (2008) Vertebral endplate trauma induces disc cell apoptosis and promotes organ degeneration in vitro. Eur Spine J 17:289–299
Bjornsson S (1998) Quantitation of proteoglycans as glycosaminoglycans in biological fluids using an alcian blue dot blot analysis. Anal Biochem 256:229–237
Pellaud J, Schote U, Arvinte T, Seelig J (1999) Conformation and self-association of human recombinant transforming growth factor-beta3 in aqueous solutions. J Biol Chem 274:7699–7704
Li J, Yoon ST, Hutton WC (2004) Effect of bone morphogenetic protein-2 (BMP-2) on matrix production, other BMPs, and BMP receptors in rat intervertebral disc cells. J Spinal Disord Tech 17:423–428
Roughley PJ (2004) Biology of intervertebral disc aging and degeneration: involvement of the extracellular matrix. Spine 29:2691–2699
Antoniou J, Steffen T, Nelson F, Winterbottom N, Hollander AP, Poole RA, Aebi M, Alini M (1996) The human lumbar intervertebral disc: evidence for changes in the biosynthesis and denaturation of the extracellular matrix with growth, maturation, ageing, and degeneration. J Clin Invest 98:996–1003
Cs-Szabo G, Ragasa-San Juan D, Turumella V, Masuda K, Thonar EJ, An HS (2002) Changes in mRNA and protein levels of proteoglycans of the anulus fibrosus and nucleus pulposus during intervertebral disc degeneration. Spine 27:2212–2219
Hutton WC, Toribatake Y, Elmer WA, Ganey TM, Tomita K, Whitesides TE (1998) The effect of compressive force applied to the intervertebral disc in vivo. A study of proteoglycans and collagen. Spine 23:2524–2537
Lu Z, Hu Y, Feng C (1998) Gene expression of fibrous main collagen in the lumbar disc. Zhonghua Wai Ke Za Zhi 36:68–71
Kuh SU, Zhu Y, Li J, Tsai KJ, Fei Q, Hutton WC, Yoon TS (2009) A comparison of three cell types as potential candidates for intervertebral disc therapy: annulus fibrosus cells, chondrocytes, and bone marrow derived cells. Joint Bone Spine 76:70–74
Kim DJ, Moon SH, Kim H, Kwon UH, Park MS, Han KJ, Hahn SB, Lee HM (2003) Bone morphogenetic protein-2 facilitates expression of chondrogenic, not osteogenic, phenotype of human intervertebral disc cells. Spine 28:2679–2684
Gilbertson L, Ahn SH, Teng PN, Studer RK, Niyibizi C, Kang JD (2008) The effects of recombinant human bone morphogenetic protein-2, recombinant human bone morphogenetic protein-12, and adenoviral bone morphogenetic protein-12 on matrix synthesis in human annulus fibrosis and nucleus pulposus cells. Spine J 8:449–456
Zhang Y, An HS, Thonar EJ, Chubinskaya S, He TC, Phillips FM (2006) Comparative effects of bone morphogenetic proteins and sox9 overexpression on extracellular matrix metabolism of bovine nucleus pulposus cells. Spine 31:2173–2179
Steck E, Bertram H, Abel R, Chen B, Winter A, Richter W (2005) Induction of intervertebral disc-like cells from adult mesenchymal stem cells. Stem Cells 23:403–411
Haberstroh K, Enz A, Zenclussen ML, Hegewald AA, Neumann K, Abbushi A, Thome C, Sittinger M, Endres M, Kaps C (2009) Human intervertebral disc-derived cells are recruited by human serum and form nucleus pulposus-like tissue upon stimulation with TGF-beta3 or hyaluronan in vitro. Tissue Cell 41(6):414–420
Chen WH, Lo WC, Lee JJ, Su CH, Lin CT, Liu HY, Lin TW, Lin WC, Huang TY, Deng WP (2006) Tissue-engineered intervertebral disc and chondrogenesis using human nucleus pulposus regulated through TGF-beta1 in platelet-rich plasma. J Cell Physiol 209:744–754
Jones MD, Pais MJ, Omiya B (1988) Bony overgrowths and abnormal calcifications about the spine. Radiol Clin North Am 26:1213–1234
Armas JB, Couto AR, Bettencourt BF (2009) Spondyloarthritis, diffuse idiopathic skeletal hyperostosis (DISH) and chondrocalcinosis. Adv Exp Med Biol 649:37–56
Kaplan FS, Shen Q, Lounev V, Seemann P, Groppe J, Katagiri T, Pignolo RJ, Shore EM (2008) Skeletal metamorphosis in fibrodysplasia ossificans progressiva (FOP). J Bone Miner Metab 26:521–530
Carragee EJ, Hurwitz EL, Weiner BK (2011) A critical review of recombinant human bone morphogenetic protein-2 trials in spinal surgery: emerging safety concerns and lessons learned. Spine J 11:471–491. doi:10.1016/j.spinee.2011.04.023
Chen NF, Smith ZA, Stiner E, Armin S, Sheikh H, Khoo LT (2010) Symptomatic ectopic bone formation after off-label use of recombinant human bone morphogenetic protein-2 in transforaminal lumbar interbody fusion. J Neurosurg Spine 12:40–46
Mindea SA, Shih P, Song JK (2009) Recombinant human bone morphogenetic protein-2-induced radiculitis in elective minimally invasive transforaminal lumbar interbody fusions: a series review. Spine (Phila Pa 1976) 34:1480–1484 discussion 1485
Crawford CH 3rd, Carreon LY, McGinnis MD, Campbell MJ, Glassman SD (2009) Perioperative complications of recombinant human bone morphogenetic protein-2 on an absorbable collagen sponge versus iliac crest bone graft for posterior cervical arthrodesis. Spine (Phila Pa 1976) 34:1390–1394
Vaidya R, Sethi A, Bartol S, Jacobson M, Coe C, Craig JG (2008) Complications in the use of rhBMP-2 in PEEK cages for interbody spinal fusions. J Spinal Disord Tech 21:557–562
Acknowledgments
The authors thank AO Foundation, Davos, Switzerland for funding, Prof. Tudor Arvinte (Therapeomics AG, Basel, Switzerland) for providing TGF-β3, Medtronic Inc. for providing BMP-2 and Ladina Ettinger Ferguson for excellent technical assistance.
Conflict of interest
None.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Haschtmann, D., Ferguson, S.J. & Stoyanov, J.V. BMP-2 and TGF-β3 do not prevent spontaneous degeneration in rabbit disc explants but induce ossification of the annulus fibrosus. Eur Spine J 21, 1724–1733 (2012). https://doi.org/10.1007/s00586-012-2371-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00586-012-2371-3