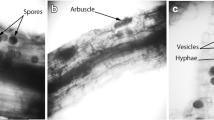
Abstract
Plant communities on Mount Segrila on the Tibetan Plateau show distinct changes at different altitudes, but little information is available on belowground communities of arbuscular mycorrhizal fungi (AMF). Root samples of two co-occurring species, Pennisetum centrasiaticum and Kobresia sp., growing in open grasslands at eight altitudes (3,446–4,556 m) were analyzed for diversity of AMF by PCR, cloning, and sequencing. Dominant plants were well colonized by AMF even at higher altitudes where spore density in rhizospheres decreased dramatically. A total of 29 operational taxonomic units (OTUs) of AMF were detected, and some novel sequence types were found. Acaulosporaceae and Glomeraceae were the dominant families. There was no significant difference in OTU richness along elevational gradients in Kobresia sp., but OTU richness in P. centrasiaticum was higher at intermediate elevations. Elevation, host plant species, and soil variables (pH, soil organic matter, and available P and N) were found to have significant effects on the overall AMF community across all elevations. Fungal community composition differed significantly between the two plant species at each elevation, and the similarity was generally higher at the intermediate elevations. No significant difference in compositional similarity was observed for Kobresia sp. with increasing elevation, but the dissimilarity increased significantly for P. centrasiaticum. These results suggest that host identity is an important determinant for the structure of the AMF communities along the elevational gradients in high altitude environments.





Similar content being viewed by others
References
Aroca R, Porcel R, Ruiz-Lozano JM (2007) How does arbuscular mycorrhizal symbiosis regulate root hydraulic properties and plasma membrane aquaporins in Phaseolus vulgaris under drought, cold or salinity stresses? New Phytol 4:808–816
Barnola LG, Montilla MG (1997) Vertical distribution of mycorrhizal colonization, root hairs, and belowground biomass in three contrasting sites from the tropical high mountains, Merida, Venezuela. Arct Alp Res 29:206–212
Becklin KM, Hertweck KL, Jumpponen A (2012) Host identity impacts rhizosphere fungal communities associated with three alpine plant species. Microb Ecol 63:682–693
Bever JD (2003) Soil community feedback and the coexistence of competitors: conceptual frameworks and empirical tests. New Phytol 157:465–473
Bjorbaekmo MFM, Carlsen T, Brysting A, Vralstad T, Hoiland K, Ugland KI, Geml J, Schumacher T, Kauserud H (2010) High diversity of root associated fungi in both alpine and arctic Dryas octopetala. BMC Plant Biol 10:244
Bryant JA, Lamanna C, Morlon H, Kerkhoff AJ, Enquist BJ, Green JL (2008) Microbes on mountain sides: contrasting elevational patterns of bacterial and plant diversity. PNAS 105:11505–11511
Cai XB, Peng YL, Feng G, Qian C (2005) AM fungi diversity and their environmental factors in altiplano grassland in Tibet. Acta Pedologica Sinica 42:642–651 (in Chinese)
Callaway RM, Brooker RW, Choler P, Kikvidze Z, Lortiek CJ, Michalet R, Paolini L, Pugnaireq FI, Newingham B, Aschehoug ET, Armasq C, Kikodze D, Cook BJ (2002) Positive interactions among alpine plants increase with stress. Nature 417:844–848
Cambardella CA, Gajda AM, Doran JW, Wienhold BJ, Kettler TA (2001) Estimation of particulate and total organic matter by weight loss on ignition. In: Lal R, Kimble JM, Follett RF, Stewart BA (eds) Assessment methods for soil carbon. Lewis, Boca Raton
Chai Y, Fan GS, Li XW, Zheng WL (2004) Study on vertical distributional belts and their floristic characters of seed plants from Shegyla Mountains of Xizang (Tibet), China. Guihuia 24:107–113 (in Chinese)
Colwell RK (2006) EstimateS: statistical estimation of species richness and shared species from samples. Version 8. Available at purl.oclc.org/estimates
Daniell TJ, Husband R, Fitter AH, Young JPW (2001) Molecular diversity of arbuscular mycorrhizal fungi colonising arable crops. FEMS Micobiol Ecol 36:203–209
Daniels BA, Skipper HD (1982) Methods for the recovery and quantitative estimation of propagules from soil. In: Schenck NC (ed) Methods and principles of mycorrhizal research. American Phytopathological Society, St. Paul, pp 29–35
de la Peña E, Rodríguez-Echeverría SR, van der Putten WH, Freitas H, Moens M (2006) Mechanism of control of root-feeding nematodes by mycorrhizal fungi in the dune grass Ammophila arenaria. New Phytol 169:829–840
Dumbrell AJ, Ashton PD, Aziz N, Feng G, Nelson M, Dytham C, Fitter AH, Helgason T (2011) Distinct seasonal assemblages of arbuscular mycorrhizal fungi revealed by massively parallel pyrosequencing. New Phytol 190:794–804
Felsenstein J (2005) PHYLIP (Phylogeny Inference Package) version 3.6. Distributed by the author. Department of Genome Sciences, University of Washington, Seattle
Fierer N, McCain CM, Meir P, Zimmermann M, Rapp JM, Silman MR, Knight R (2011) Microbes do not follow the elevational diversity patterns of plants and animals. Ecology 92:797–804
Fitter AH (2005) Darkness visible: reflections on underground ecology. J Ecol 93:231–243
Gai JP, Cai XB, Feng G, Christie P, Li XL (2006) Arbuscular mycorrhizal fungi associated with sedges on the Tibetan plateau. Mycorrhiza 16:151–157
Gai JP, Christie P, Cai XB, Fan JQ, Zhang JL, Feng G, Li XL (2009) Occurrence and distribution of arbuscular mycorrhizal fungal species in three types of grassland community of the Tibetan Plateau. Ecol Res 24:1345–1350
Gai JP, Tian H, Yang FY, Christie P, Li XL, Klironomos JN (2012) Arbuscular mycorrhizal fungal diversity along a Tibetan elevation gradient. Pedobiologia 55:145–151
Goslee SC, Urban DL (2007) The Ecodist package for dissimilarity-based analysis of ecological data. J Stat Softw 22:1–19
Hijri I, Sykorova Z, Oehl F, Ineichen K, Mader P, Wiemken A, Redecker D (2006) Communities of arbuscular mycorrhizal fungi in arable soils are not necessarily low in diversity. Mol Ecol 15:2277–2289
Jakobsen I, Abbott LK, Robson AD (1992) External hyphae of vesicular-arbuscular mycorrhizal fungi associated with Trifolium subterraneum L. 1. Spread of hyphae and phosphorus inflow into roots. New Phytol 120:509–516
Kiers ET, Duhamel M, Beesetty Y, Mensah JA, Franken O, Verbruggen E, Fellbaum CR, Kowalchuk GA, Hart MM, Bago A, Palmer TM, West SA, Vandenkoornhuyse P, Jansa J, Bücking H (2011) Reciprocal rewards stabilize cooperation in the mycorrhizal symbiosis. Science 333:880–882
Körner C (2003) Carbon limitation in trees. J Ecol 91:4–17
Koske RE (1987) Distribution of VA mycorrhizal fungi along a latitudinal temperature gradient. Mycologia 79:55–68
Krüger M, Stockinger H, Krüger C, Schüßler A (2009) DNA-based species level detection of Glomeromycota: one PCR primer set for all arbuscular mycorrhizal fungi. New Phytol 183:212–223
Li MG (2000) Operating principles and techniques of plant genes. Tianjin Science and Technology Press, Tianjin
Li LF, Li T, Zhao ZW (2009) Molecular diversity of arbuscular mycorrhizal fungi and their distribution patterns related to host: plants and habitats in a hot and arid ecosystem, southwest China. FEMS Microbiol Ecol 71:418–427
Liu Y, He L, An L, Helgason T, Feng H (2009) Arbuscular mycorrhizal dynamics in a chronosequence of Caragana korshinskii plantations. FEMS Microbiol Ecol 67:81–92
Liu Y, He J, Shi G, An L, Opik M, Feng H (2011) Diverse communities of arbuscular mycorrhizal fungi inhabit sites with very high altitude in Tibet Plateau. FEMS Microbial Ecol 78:355–365
Lovelock CE, Ewel JJ (2005) Links between tree species, symbiotic fungal diversity and ecosystem functioning in simplified tropical ecosystems. New Phytol 167:219–228
Lugo MA, Ferrero M, Menoyo E, Menoyo E, Estévez MC, Siñeriz F, Anton A (2008) Arbuscular mycorrhizal fungi and rhizospheric bacteria diversity along an altitudinal gradient in South American Puna Grassland. Microb Ecol 55:705–713
Muehlmann O, Peintner U (2008) Ectomycorrhiza of Kobresia myosuroides at a primary successional glacier forefront. Mycorrhiza 18:355–362
Mullen RB, Schmidt SK (1993) Mycorrhizal infection, phosphorus uptake, and phenology in Ranunculus adoneus: implications for the functioning of mycorrhizae in alpine systems. Oecologia 94:229–234
Muthukumar T, Udaiyan K, Shanmughavel P (2004) Mycorrhiza insedges: an overview. Mycorrhiza 14:65–77
Nara K, Hogetsu T (2004) Ectomycorrhizal fungi on established shrubs facilitate subsequent seedling establishment of successional plant species. Ecology 85:1700–1707
Oehl F, Sýkorová Z, Redecker D, Wiemken A, Sieverding E (2006) Acaulospora alpina, a new arbuscular mycorrhizal fungal species characteristic for high mountainous and alpine regions of the Swiss Alps. Mycologia 98:286–294
Oehl F, Sieverding E, Inerchen K, Maeder P, Wiemken A, Boller T (2009) Distinct sporulation dynamics of arbuscular mycorrhizal fungal communities from different agroecosystems in long-term microcosms. Agric Ecosyst Environ 134:257–268
Olsen SR, Sommers LE (1982) Phosphorus. In: Page AL, Miller RH, Keeney DR (eds) Methods of soil analysis. Part 2. American Society of Agronomy, Madison
Phillips J, Hayman D (1970) Improved procedures for cleaning and staining parasitic and vesicular arbuscular mycorrhizal fungi for rapid assessment of infection. Trans Br Mycol Soc 55:158–160
Read DJ, Haselwandter K (1981) Observations on the mycorrhizal status of some alpine plant communities. New Phytol 88:341–352
Rehfeldt GE (1994) Adaptation of Picea engelmannii populations to the heterogeneous environments of the Intermountain West Canadian. J Bot 72:1197–1208
Rillig MC, Wright SF, Shaw MR, Field CB (2002) Artificial climate warming positively affects arbuscular mycorrhizae but decreases soil aggregate water stability in an annual grassland. Oikos 97:52–58
Santos-González JC, Finlay RD, Tehler A (2007) Seasonal dynamics of arbuscular mycorrhizal fungal communities in roots in a semi-natural grassland. Appl Environ Microbiol 73:5613–5623
Schadt CW, Schmidt SK (2001) Characterization of the ectomycorrhizal fungi associated with Kobresia myosuroides. Phytopathology 91:S122
Schloss PD, Handelsman J (2005) Introducing DOTUR, a computer program for defining operational taxonomic units and estimating species richness. Appl Environ Microbiol 71:1501–1506
Schmidt SK, Sobieniak-Wiseman LC, Kageyama SA, Halloy SRP, Schadt CW (2008) Mycorrhizal and dark-Septate fungi in plant roots above 4270 meters elevation in the Andes and Rocky Mountains. Arct Antarct Alp Res 40:576–583
Smith SE, Read DJ (2008) Mycorrhizal symbiosis. Academic, Cambridge
Sworfford DL (2003) Paup* phylogenetic analysis using parsimony (* and other methods), Version 4.0b10. Sinauer Associates, Sunderland
ter Braak CJF, Šmilauer P (2002) CANOCO reference manual and CanoDraw for Windows user's guide: software for Canonical Community Ordination (version 4.5). Microcomputer Power, Ithaca
Thompson JD, Gibson TJ, Plewniak F, Jeanmougin F, Higgins DG (1997) The CLUSTAL_X Windows interface: flexible strategies for multiple sequence alignment aided by quality analysis tools. Nucleic Acids Res 25:4876–4882
Trouvelot A, Kough J, Gianinazzi-Pearson V (1986) Mesure dutaux de mycorrhization VA d’un systeme radiculaire. Recherche de methods d’estimation ayant une signification functionnelle. In: Gianinazzi-Pearson V, Gianinazzi S (eds) Physiological and genetic aspects of mycorrhizae. INRA, Paris, pp 217–221
van der Heijden MGA, Boller T, Wiemken A (1998a) Different arbuscular mycorrhizal fungal species are potential determinants of plant community structure. Ecology 79:2082–2091
van der Heijden MGA, Klironomos JN, Ursic M (1998b) Mycorrhizal fungal diversity determines plant biodiversity, ecosystem variability and productivity. Nature 396:69–72
Vandenkoornhuyse P, Husband R, Daniell TJ (2002) Arbuscular mycorrhizal community composition associated with two plant species in a grassland ecosystem. Mol Ecol 11:1555–1564
Wagg C, Brian C, Husband D, Green S, Hugues B, Massicotte R, Peterson L (2011) Soil microbial communities from an elevational cline differ in their effect on conifer seedling growth. Plant Soil 340:491–504
Wang B, Qiu YL (2006) Phylogenetic distribution and evolution of mycorrhizas in land plants. Mycorrhiza 16:299–363
Yang ZL, Gao Q (2010) Ectomycorrhizal fungi associated with two species of Kobresia in an alpine meadow in the eastern Himalaya. Mycorrhiza 20:281–287
Acknowledgments
Financial support from the National Natural Science Foundation of China (Grant Nos. 21020106, 41161043, and the Innovative Group Grant 31121062) and PhD Programs Foundation of the Ministry of Education of China (20100008110003) is acknowledged. We also thank Professor John Klironomos, University of British Columbia–Okanagan, Canada, for initiating the project. We are grateful to the anonymous reviewers for their helpful comments on an earlier version of this manuscript.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Li, X., Gai, J., Cai, X. et al. Molecular diversity of arbuscular mycorrhizal fungi associated with two co-occurring perennial plant species on a Tibetan altitudinal gradient. Mycorrhiza 24, 95–107 (2014). https://doi.org/10.1007/s00572-013-0518-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00572-013-0518-7