Abstract
Background
Hepatocellular carcinoma (HCC) is one of the most common solid tumors worldwide. Surgery is potentially curative, but high recurrence rates worsen patient prognosis. The interaction between the proteins programmed cell death 1 (PD-1) and programmed cell death 1 ligand 1 (PD-L1) is an important immune checkpoint. The significance of PD-L1 expression and human leukocyte antigen class I (HLA class I), recognized by CD8 T cells, in the prognosis of patients with HCC remains to be determined.
Methods
We assessed the levels of PD-L1 and HLA class I expression on HCC samples from 80 patients who had undergone hepatectomy at our institution, and evaluated the correlations between PD-L1 and HLA class I expression and patient prognosis.
Results
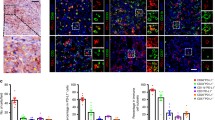
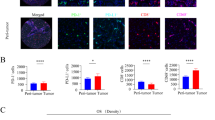
High HLA class I expression was correlated with significantly better recurrence-free survival (RFS), but not overall survival (OS). Multivariate analysis showed that high HLA class I expression was an independent predictor of improved RFS. Low expression of PD-L1 on HCC tended to predict better OS, but the difference was not statistically significant. PD-L1 expression on HCC correlated with the number of CD163-positive macrophages and HLA class I expression with CD3-positive cell infiltration. Univariable and multivariable analyses showed that combined PD-L1 low/HLA class I high expression on HCCs was prognostic for improved OS and RFS.
Conclusions
PD-L1 status may be a good predictor of prognosis in HCC patients with high HLA class I expression. Novel therapies targeting the PD-L1/PD-1 pathway may improve the prognosis of patients with HCC.





Similar content being viewed by others
Abbreviations
- HCC:
-
Hepatocellular carcinoma
- PD-1:
-
Programmed cell death 1
- PD-L1:
-
Programmed cell death 1 ligand 1
- MHC:
-
Major histocompatibility complex
- HLA:
-
Human leukocyte antigen
- OS:
-
Overall survival
- RFS:
-
Recurrence-free survival
- pSTAT3:
-
Phosphorylated signal transducer and activator of transcription 3
- TAM:
-
Tumor-associated macrophages
References
Shirabe K, Kanematsu T, Matsumata T, et al. Factors linked to early recurrence of small hepatocellular carcinoma after hepatectomy: univariate and multivariate analyses. Hepatology. 1991;14:802–5.
Herr I, Schemmer P, Buchler MW. On the TRAIL to therapeutic intervention in liver disease. Hepatology. 2007;46:266–74.
Gao Q, Qiu SJ, Fan J, et al. Intratumoral balance of regulatory and cytotoxic T cells is associated with prognosis of hepatocellular carcinoma after resection. J Clin Oncol: Off J Am Soc Clin Oncol. 2007;25:2586–93.
Bricard G, Bouzourene H, Martinet O, et al. Naturally acquired MAGE-A10- and SSX-2-specific CD8+ T cell responses in patients with hepatocellular carcinoma. J Immunol. 2005;174:1709–16.
Steel JL, Geller DA, Gamblin TC, et al. Depression, immunity, and survival in patients with hepatobiliary carcinoma. J Clin Oncol: Off J Am Soc Clin Oncol. 2007;25:2397–405.
Riley JL. PD-1 signaling in primary T cells. Immunol Rev. 2009;229:114–25.
Okazaki T, Honjo T. The PD-1-PD-L pathway in immunological tolerance. Trends Immunol. 2006;27:195–201.
Dong H, Strome SE, Salomao DR, et al. Tumor-associated B7-H1 promotes T-cell apoptosis: a potential mechanism of immune evasion. Nat Med. 2002;8:793–800.
Shi F, Shi M, Zeng Z, et al. PD-1 and PD-L1 upregulation promotes CD8(+) T-cell apoptosis and postoperative recurrence in hepatocellular carcinoma patients. Int J Cancer (J International du Cancer). 2011;128:887–96.
Topalian SL, Hodi FS, Brahmer JR, et al. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. New Engl J Med. 2012;366:2443–54.
Brahmer JR, Tykodi SS, Chow LQ, et al. Safety and activity of anti-PD-L1 antibody in patients with advanced cancer. New Engl J Med. 2012;366:2455–65.
Kuang DM, Zhao Q, Peng C, et al. Activated monocytes in peritumoral stroma of hepatocellular carcinoma foster immune privilege and disease progression through PD-L1. J Exp Med. 2009;206:1327–37.
Gao Q, Wang XY, Qiu SJ, et al. Overexpression of PD-L1 significantly associates with tumor aggressiveness and postoperative recurrence in human hepatocellular carcinoma. Clin Cancer Res: Off J Am Assoc Cancer Res. 2009;15:971–9.
Campoli M, Ferrone S. HLA antigen changes in malignant cells: epigenetic mechanisms and biologic significance. Oncogene. 2008;27:5869–85.
Hamanishi J, Mandai M, Iwasaki M, et al. Programmed cell death 1 ligand 1 and tumor-infiltrating CD8+ T lymphocytes are prognostic factors of human ovarian cancer. Proc Natl Acad Sci USA. 2007;104:3360–5.
Garrido F, Cabrera T, Aptsiauri N. “Hard” and “soft” lesions underlying the HLA class I alterations in cancer cells: implications for immunotherapy. Int J Cancer (J International du Cancer). 2010;127:249–56.
Mano Y, Aishima S, Fujita N, et al. Tumor-associated macrophage promotes tumor progression via STAT3 signaling in hepatocellular carcinoma. Pathobiol: J Immunopathol Mol Cell Biol. 2013;80:146–54.
Pardoll DM. The blockade of immune checkpoints in cancer immunotherapy. Nat Rev Cancer. 2012;12:252–64.
Bigelow E, Bever KM, Xu H, et al. Immunohistochemical staining of B7-H1 (PD-L1) on paraffin-embedded slides of pancreatic adenocarcinoma tissue. J Vis Exp. 2013;71:e4059.
Watson NF, Ramage JM, Madjd Z, et al. Immunosurveillance is active in colorectal cancer as downregulation but not complete loss of MHC class I expression correlates with a poor prognosis. Int J Cancer (J International du Cancer). 2006;118:6–10.
Dunn GP, Koebel CM, Schreiber RD. Interferons, immunity and cancer immunoediting. Nat Rev Immunol. 2006;6:836–48.
Cicinnati VR, Zhang X, Yu Z, et al. Increased frequencies of CD8+ T lymphocytes recognizing wild-type p53-derived epitopes in peripheral blood correlate with presence of epitope loss tumor variants in patients with hepatocellular carcinoma. Int J Cancer (J International du Cancer). 2006;119:2851–60.
Butterfield LH, Meng WS, Koh A, et al. T cell responses to HLA-A*0201-restricted peptides derived from human alpha fetoprotein. J Immunol. 2001;166:5300–8.
Matsui M, Machida S, Itani-Yohda T, et al. Downregulation of the proteasome subunits, transporter, and antigen presentation in hepatocellular carcinoma, and their restoration by interferon-gamma. J Gastroenterol Hepatol. 2002;17:897–907.
Nishibe M, Une Y, Kobayashi M, et al. HLA class I antigens are possible prognostic factors in hepatocellular carcinoma. Int J Oncol. 1996;8:1243–7.
Tang X. Tumor-associated macrophages as potential diagnostic and prognostic biomarkers in breast cancer. Cancer Lett. 2013;332:3–10.
Acknowledgments
This work was supported by a Grant-in-Aid from the Japan Society for the Promotion of Science (to Shirabe Ken; #24390320).
Conflict of interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Additional information
Y. Umemoto and S. Okano contributed equally to this work.
Electronic supplementary material
Below is the link to the electronic supplementary material.
535_2014_933_MOESM1_ESM.pptx
Supplementary material 1 Fig. S1 (A, B) Sections were cut 4 μm thick, deparaffinized in xylene, and dehydrated in an ethanol series. For antigen retrieval, the specimens were pretreated in an autoclave in 0.01 M citrate buffer (pH 6.0). After blocking nonspecific binding of antibodies, the specimens were incubated with anti-HLA class 1 (EMR8-5, 1:500; MBL, Tokyo, Japan) followed by Alexa 647-conjugated anti-mouse IgG1 mAb, and with anti-CD3 (polyclonal rabbit antibody, Dako, Glostrup, Denmark) followed by Alexa 555-conjugated anti-rabbit antibody, each for 60 min at room temperature. The samples were subsequently, and counterstained with DAPI/anti-fade mounting medium (ProLong® Gold antifade reagent with DAPI, Life Technologies, Carlsbad, CA). Fluorescence was detected by confocal microscopy A1 (Nikon Instruments Inc., Tokyo, Japan). HLA class I was highly expressed on the hepatoma cell membranes of patient no. 1159, with this tumor showing prominent infiltration of CD3-positive T cells (A, original magnification × 200). HLA class I was hardly detected on the hepatoma cell membranes, but not on the endothelial cells, of patient no. 1164, with this tumor showing minimal infiltration of CD3-positive T cells (B, upper photograph, original magnification ×100). HLA class I was detected on the hepatoma cell membranes of another the site in patient L1164, with this area showing infiltration of CD3-positive T (B, lower photograph, original magnification ×100). (C) Tumor tissues were embedded in TissueTek cutting medium (Sakura Finetek, Tokyo, Japan) using Cryomold (Sakura Finetek), and 6-μm frozen tissue sections were fixed with cold acetone, washed with PBS, and incubated with 5 % skim milk in PBS for 1 h to blocking nonspecific binding. The sections were immunostained with phycoerythrin (PE)-conjugated anti-PD-L1 mAb (Biolegend, San Diego, CA), FITCconjugated HLA class I (BD Biosciences, San Diego, CA), and allophycocyanin (Apc)-conjugated CD163 mAb (Biolegend), and counterstained with DAPI/anti-fade mounting medium (ProLong® Gold antifade reagent with DAPI, Life Technologies). The sections were examined by microscopy (Biozero BZ-8000, KEYENCE Japan, Osaka, Japan), and composite images were created using the attached software. Hepatoma cells expressing PD-L1 (encircled area) were present at HCC sites infiltrated by CD163-positive tumor associated macrophages (arrow head). (PPTX 5910 kb)
535_2014_933_MOESM2_ESM.pptx
Supplementary material 2 Fig. S2 Sections showing HCC specimens derived from patients No.1875 (A, B, E, F; PD-L high/HLA class I high) and 1959 (C, D, G, H; PD-L1 low/HLA class I high) stained with H&E (A-D) and antibody to CD3 (E–H). Degenerative changes, necrosis, and necrosis of the HCC were prominent (arrow, C, D) and associated with massive infiltration of CD3-positive cells (G, H) in patient 1959, but were minimal (A, B) and associated with infiltration of a few CD3-positive cells only in the peripheral tissue of the tumor in patient 1875 (E, F). Despite similar characteristics of the tumor factors, patient 1959 showed no evidence of tumor recurrence, whereas patient 1959 showed HCC recurrence 180 days after curative hepatectomy. (PPTX 9249 kb)
Rights and permissions
About this article
Cite this article
Umemoto, Y., Okano, S., Matsumoto, Y. et al. Prognostic impact of programmed cell death 1 ligand 1 expression in human leukocyte antigen class I-positive hepatocellular carcinoma after curative hepatectomy. J Gastroenterol 50, 65–75 (2015). https://doi.org/10.1007/s00535-014-0933-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00535-014-0933-3