Abstract
Background
Barrett’s esophagus is characterized by a distinct Th-2-predominant cytokine profile, unlike the pro-inflammatory nature of reflux esophagitis. The aim of this study was to examine the role of Th-2 cytokines during the development of Barrett’s esophagus, using a rat model.
Methods
Barrett’s esophagus was induced by Levrat’s esophagojejunostomy technique in Brown-Norway (BN) rats. Rats were killed at 8, 15, 30, and 50 weeks after the operation. We studied the incidences of esophagitis and Barrett’s esophagus, and the mRNA expression of cytokines and CDX2 by real-time reverse transcriptase-polymerase chain reaction and immunohistochemical staining. Finally, we compared the incidence of Barrett’s esophagus in BN rats with that in Sprague-Dawley (SD) rats.
Results
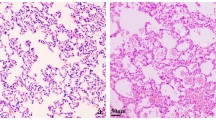
Esophagitis was found in all rats. Barrett’s esophagus appeared 8 weeks after the operation, and its incidence and length increased over time. Levels of Th-2 cytokines such as interleukin (IL)-4, IL-10, and IL-13 were significantly increased in Barrett’s esophagus as compared to those in non-Barrett’s esophagus, while there were no differences in the levels of pro-inflammatory cytokines. The peak of elevated IL-4 mRNA was observed before that of CDX2 mRNA. IL-4 was co-localized in CD4-positive cells and CDX2-positive goblet cells. The incidence of Barrett’s esophagus was more common in BN rats (8/10, 80%) than in SD rats (2/7, 28%) at 30 weeks.
Conclusion
Th-2 cytokines, especially IL-4, may play a crucial role in the development of Barrett’s esophagus in an early phase. These results provide understanding of the pathogenesis of Barrett’s esophagus from the aspect of the Th-2 immune response.






Similar content being viewed by others
References
Champion G, Richter JE, Vaezi MF, Singh S, Alexander R. Duodenogastroesophageal reflux: relationship to pH and importance in Barrett’s esophagus. Gastroenterology. 1994;107:747–54.
Vaezi MF, Richter JE. Role of acid and duodenogastroesophageal reflux in gastroesophageal reflux disease. Gastroenterology. 1996;111:1192–9.
Falk GW. Barrett’s esophagus. Gastroenterology. 2002;122:1569–91.
Jankowski JA, Wright NA, Meltzer SJ, Triadafilopoulos G, Geboes K, Casson AG, et al. Molecular evolution of the metaplasia-dysplasia-adenocarcinoma sequence in the esophagus. Am J Pathol. 1999;154:965–73.
Pera M. Experimental Barrett’s esophagus and the origin of intestinal metaplasia. Chest Surg Clin N Am. 2002;12:25–37.
Seery JP. Stem cells of the oesophageal epithelium. J Cell Sci. 2002;115:1783–9.
Sarosi G, Brown G, Jaiswal K, Feagins LA, Lee E, Crook TW, et al. Bone marrow progenitor cells contribute to esophageal regeneration and metaplasia in a rat model of Barrett’s esophagus. Dis Esophagus. 2008;21:43–50.
Souza RF, Krishnan K, Spechler SJ. Acid, bile, and CDX: the ABCs of making Barrett’s metaplasia. Am J Physiol Gastrointest Liver Physiol. 2008;295:G211–8.
Hu Y, Williams VA, Gellersen O, Jones C, Watson TJ, Peters JH. The pathogenesis of Barrett’s esophagus: secondary bile acids upregulate intestinal differentiation factor CDX2 expression in esophageal cells. J Gastrointest Surg. 2007;11:827–34.
Kazumori H, Ishihara S, Kinoshita Y. Roles of caudal-related homeobox gene Cdx1 in oesophageal epithelial cells in Barrett’s epithelium development. Gut. 2009;58:620–8.
Souza RF, Huo X, Mittal V, Schuler CM, Carmack SW, Zhang HY, et al. Gastroesophageal reflux might cause esophagitis through a cytokine-mediated mechanism rather than caustic acid injury. Gastroenterology. 2009;137:1776–84.
Fitzgerald RC, Onwuegbusi BA, Bajaj-Elliott M, Saeed IT, Burnham WR, Farthing MJ. Diversity in the oesophageal phenotypic response to gastro-oesophageal reflux: immunological determinants. Gut. 2002;50:451–9.
Moons LM, Kusters JG, Bultman E, Kuipers EJ, van Dekken H, Tra WM, et al. Barrett’s oesophagus is characterized by a predominantly humoral inflammatory response. J Pathol. 2005;207:269–76.
Dvorakova K, Payne CM, Ramsey L, Holubec H, Sampliner R, Dominguez J, et al. Increased expression and secretion of interleukin-6 in patients with Barrett’s esophagus. Clin Cancer Res. 2004;10:2020–8.
Pera M, Brito MJ, Poulsom R, Riera E, Grande L, Hanby A, et al. Duodenal-content reflux esophagitis induces the development of glandular metaplasia and adenosquamous carcinoma in rats. Carcinogenesis. 2000;21:1587–91.
Levrat M, Lambert R, Kirshbaum G. Esophagitis produced by reflux of duodenal contents in rats. Am J Dig Dis. 1962;7:564–73.
Pera M, Cardesa A, Bombi JA, Ernst H, Pera C, Mohr U. Influence of esophagojejunostomy on the induction of adenocarcinoma of the distal esophagus in Sprague-Dawley rats by subcutaneous injection of 2,6-dimethylnitrosomorpholine. Cancer Res. 1989;49:6803–8.
Sugawa T, Fujiwara Y, Yamagami H, Watanabe K, Tanigawa T, Shiba M, et al. A novel rat model to determine interaction between reflux oesophagitis and bronchial asthma. Gut. 2008;57:575–81.
Hamaguchi M, Fujiwara Y, Takashima T, Hayakawa T, Sasaki E, Shiba M, et al. Increased expression of cytokines and adhesion molecules in rat chronic esophagitis. Digestion. 2003;68:189–97.
Wang KK, Sampliner RE. Updated guidelines 2008 for the diagnosis, surveillance and therapy of Barrett’s esophagus. Am J Gastroenterol. 2008;103:788–97.
Watson A, Shepherd NA. The definition of “Barrett’s” columnar-lined oesophagus. Guidelines for the diagnosis and management of Barrett’s columnar-lined oesophagus: a report of the Working Party of the British Society of Gastroenterology. Loughborough: Q3 Print Project Management Limited; 2005. p. 4–6.
Hellier MD, Shepherd NA. Diagnosis of columnar-lined oesophagus. Guidelines for the diagnosis and management of Barrett’s columnar-lined oesophagus: a report of the Working Party of the British Society of Gastroenterology. Loughborough: Q3 Print Project Management Limited; 2005. p. 13–7.
Japan Esophageal Society. Japanese classification of esophageal cancer. 10th ed. Tokyo: Kanahara & Co., Ltd.; 2008. p. 42–4.
Kuperman D, Schofield B, Wills-Karp M, Grusby MJ. Signal transducer and activator of transcription factor 6 (Stat6)-deficient mice are protected from antigen-induced airway hyperresponsiveness and mucus production. J Exp Med. 1998;187:939–48.
Gavett SH, O’Hearn DJ, Karp CL, Patel EA, Schofield BH, Finkelman FD, et al. Interleukin-4 receptor blockade prevents airway responses induced by antigen challenge in mice. Am J Physiol. 1997;272:L253–61.
Dabbagh K, Takeyama K, Lee HM, Ueki IF, Lausier JA, Nadel JA. IL-4 induces mucin gene expression and goblet cell metaplasia in vitro and in vivo. J Immunol. 1999;162:6233–7.
Temann UA, Prasad B, Gallup MW, Basbaum C, Ho SB, Flavell RA, et al. A novel role for murine IL-4 in vivo: induction of MUC5AC gene expression and mucin hypersecretion. Am J Respir Cell Mol Biol. 1997;16:471–8.
Iwashita J, Sato Y, Sugaya H, Takahashi N, Sasaki H, Abe T. mRNA of MUC2 is stimulated by IL-4, IL-13 or TNF-alpha through a mitogen-activated protein kinase pathway in human colon cancer cells. Immunol Cell Biol. 2003;81:275–82.
Dohi T, Fujihashi K, Koga T, Shirai Y, Kawamura YI, Ejima C, et al. T helper type-2 cells induce ileal villus atrophy, goblet cell metaplasia, and wasting disease in T cell-deficient mice. Gastroenterology. 2003;124:672–82.
Kazumori H, Ishihara S, Rumi MA, Kadowaki Y, Kinoshita Y. Bile acids directly augment caudal related homeobox gene Cdx2 expression in oesophageal keratinocytes in Barrett’s epithelium. Gut. 2006;55:16–25.
Liu T, Zhang X, So CK, Wang S, Wang P, Yan L, et al. Regulation of Cdx2 expression by promoter methylation, and effects of Cdx2 transfection on morphology and gene expression of human esophageal epithelial cells. Carcinogenesis. 2007;28:488–96.
Pera M, de Bolos C, Brito MJ, Palacin A, Grande L, Cardesa A, et al. Duodenal-content reflux into the esophagus leads to expression of Cdx2 and Muc2 in areas of squamous epithelium in rats. J Gastrointest Surg. 2007;11:869–74.
Tatsuta T, Mukaisho K, Sugihara H, Miwa K, Tani T, Hattori T. Expression of Cdx2 in early GRCL of Barrett’s esophagus induced in rats by duodenal reflux. Dig Dis Sci. 2005;50:425–31.
Ingravallo G, Dall’Olmo L, Segat D, Fassan M, Mescoli C, Dazzo E, et al. CDX2 hox gene product in a rat model of esophageal cancer. J Exp Clin Cancer Res. 2009;28:108.
Zhang HY, Zhang X, Chen X, Thomas D, Hormi-Carver K, Elder F, et al. Differences in activity and phosphorylation of MAPK enzymes in esophageal squamous cells of GERD patients with and without Barrett’s esophagus. Am J Physiol Gastrointest Liver Physiol. 2008;295:G470–8.
Huo X, Zhang HY, Zhang XI, Lynch JP, Strauch ED, Wang JY, et al. Acid and bile salt-induced CDX2 expression differs in esophageal squamous cells from patients with and without Barrett’s esophagus. Gastroenterology. 2010;139:194–203.
Hylkema MN, Hoekstra MO, Luinge M, Timens W. The strength of the OVA-induced airway inflammation in rats is strain dependent. Clin Exp Immunol. 2002;129:390–6.
Yang L, Lu X, Nossa CW, Francois F, Peek RM, Pei Z. Inflammation and intestinal metaplasia of the distal esophagus are associated with alterations in the microbiome. Gastroenterology. 2009;137:588–97.
Acknowledgments
This study was supported in part by a Grant-in-Aid for Scientific Research from the Ministry of Education, Culture, Sports, Science and Technology of Japan.
Conflict of interest
No conflicts of interest exist.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kohata, Y., Fujiwara, Y., Machida, H. et al. Role of Th-2 cytokines in the development of Barrett’s esophagus in rats. J Gastroenterol 46, 883–893 (2011). https://doi.org/10.1007/s00535-011-0405-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00535-011-0405-y