Abstract
Purpose
The objective of this study was to describe the incidence of grade 3/4 neutropenia, patterns of chemotherapy treatment, and granulocyte colony-stimulating factor (G-CSF) use patterns among patients with non-Hodgkin’s lymphoma (NHL) <65 and ≥65 years.
Methods
This retrospective, observational study included adult patients with NHL who received cyclophosphamide, doxorubicin, vincristine, and prednisone ± rituximab (CHOP ± R) from January 2006 to June 2010.
Results
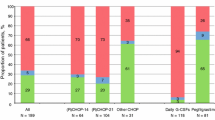
A total of 1,579 patients were included, with 54.1 % <65 years and 45.9 % ≥65 years. Most received CHOP-R on a Q3W schedule. Among patients <65 years, the incidence of grade 3/4 neutropenia was 52.3 %, the mean relative dose intensity (RDI) was 80.4 %, and the incidences of dose delays and reductions were 26.5 and 9.6 %, respectively. Among patients ≥65 years, the incidence of grade 3/4 neutropenia was 63.2 %, the mean RDI was 73.9 %, and the incidences of dose delays and reductions were 24.6 and 24.9 %, respectively. Most patients (86.9 %) received G-CSF. Among patients <65 years, 71.9, 17.4, and 10.7 % first received G-CSF as primary prophylaxis, secondary prophylaxis, or treatment, respectively. Among patients ≥65 years, 80.1, 11.6, and 8.3 % first received G-CSF as primary prophylaxis, secondary prophylaxis, or treatment, respectively.
Conclusions
Chemotherapy regimens and schedules were similar among age groups. Grade 3/4 neutropenia, reduced RDI, and dose delays were common in both age groups, though patients ≥65 years had a higher incidence of dose reductions. In spite of these similarities, patients <65 years were less likely to receive primary prophylactic G-CSF. Thus, careful assessment of neutropenia risk factors is needed across age groups to determine appropriate G-CSF use and support planned chemotherapy.



Similar content being viewed by others
References
Howlader N, Noone A, Krapcho M, Neyman N, Aminou R, Waldron W, Altekruse S, Kosary C, Ruhl J, Tatalovich Z, Cho H, Mariotto A, Eisner M, Lewis D, Chen H, Feuer E, Cronin K, Edwards B (2011) SEER cancer statistics review, 1975–2008. National Cancer Institute, Bethesda
Altekruse SF, Kosary CL, Krapcho M, Neyman N, Aminou R, Waldron W, Ruhl J, Howlader N, Tatalovich Z, Cho H, Mariotto A, Eisner MP, Lewis DR, Cronin K, Chen HS, Feuer EJ, Stinchcomb DG, Edwards BK (2010) SEER cancer statistics review, 1975–2007. National Cancer Institute, Bethesda
Armitage JO, Weisenburger DD (1998) New approach to classifying non-Hodgkin's lymphomas: clinical features of the major histologic subtypes. Non-Hodgkin's Lymphoma Classification Project. J Clin Oncol 16(8):2780–2795
National Comprehensive Cancer Network (2011) NCCN Clinical Practice Guidelines in Oncology: Non-Hodgkin's Lymphomas version 2.2011. National Comprehensive Cancer Network, Fort Washington, PA
Coiffier B, Lepage E, Briere J, Herbrecht R, Tilly H, Bouabdallah R, Morel P, Van Den Neste E, Salles G, Gaulard P, Reyes F, Lederlin P, Gisselbrecht C (2002) CHOP chemotherapy plus rituximab compared with CHOP alone in elderly patients with diffuse large-B-cell lymphoma. N Engl J Med 346(4):235–242. doi:10.1056/NEJMoa011795
Feld R, Bodey GP (1977) Infections in patients with malignant lymphoma treated with combination chemotherapy. Cancer 39(3):1018–1025
Lyman GH, Morrison VA, Dale DC, Crawford J, Delgado DJ, Fridman M (2003) Risk of febrile neutropenia among patients with intermediate-grade non-Hodgkin's lymphoma receiving CHOP chemotherapy. Leuk Lymphoma 44(12):2069–2076. doi:10.1080/1042819031000119262
Bodey GP, Buckley M, Sathe YS, Freireich EJ (1966) Quantitative relationships between circulating leukocytes and infection in patients with acute leukemia. Ann Intern Med 64(2):328–340
Crawford J, Dale DC, Lyman GH (2004) Chemotherapy-induced neutropenia: risks, consequences, and new directions for its management. Cancer 100(2):228–237
Bosly A, Bron D, Van Hoof A, De Bock R, Berneman Z, Ferrant A, Kaufman L, Dauwe M, Verhoef G (2008) Achievement of optimal average relative dose intensity and correlation with survival in diffuse large B-cell lymphoma patients treated with CHOP. Ann Hematol 87(4):277–283
Epelbaum R, Haim N, Ben-Shahar M, Ron Y, Cohen Y (1988) Dose-intensity analysis for CHOP chemotherapy in diffuse aggressive large cell lymphoma. Isr J Med Sci 24(9–10):533–538
Kwak LW, Halpern J, Olshen RA, Horning SJ (1990) Prognostic significance of actual dose intensity in diffuse large-cell lymphoma: results of a tree-structured survival analysis. J Clin Oncol 8(6):963–977
Caggiano V, Weiss RV, Rickert TS, Linde-Zwirble WT (2005) Incidence, cost, and mortality of neutropenia hospitalization associated with chemotherapy. Cancer 103(9):1916–1924. doi:10.1002/cncr.20983
Dulisse B, Li X, Gayle JA, Barron RL, Ernst FR, Rothman KJ, Legg JC, Kaye JA (2013) A retrospective study of the clinical and economic burden during hospitalizations among cancer patients with febrile neutropenia. J Med Econ. doi:10.3111/13696998.2013.782034
Kuderer NM, Dale DC, Crawford J, Cosler LE, Lyman GH (2006) Mortality, morbidity, and cost associated with febrile neutropenia in adult cancer patients. Cancer 106(10):2258–2266. doi:10.1002/cncr.21847
Gisselbrecht C, Haioun C, Lepage E, Bastion Y, Tilly H, Bosly A, Dupriez B, Marit G, Herbrecht R, Deconinck E, Marolleau JP, Yver A, Dabouz-Harrouche F, Coiffier B, Reyes F (1997) Placebo-controlled phase III study of lenograstim (glycosylated recombinant human granulocyte colony-stimulating factor) in aggressive non-Hodgkin's lymphoma: factors influencing chemotherapy administration. Groupe d'Etude des Lymphomes de l'Adulte. Leuk Lymphoma 25(3–4):289–300
Lyman GH, Kuderer NM, Djulbegovic B (2002) Prophylactic granulocyte colony-stimulating factor in patients receiving dose-intensive cancer chemotherapy: a meta-analysis. Am J Med 112(5):406–411
Pettengell R, Gurney H, Radford JA, Deakin DP, James R, Wilkinson PM, Kane K, Bentley J, Crowther D (1992) Granulocyte colony-stimulating factor to prevent dose-limiting neutropenia in non-Hodgkin's lymphoma: a randomized controlled trial. Blood 80(6):1430–1436
Zinzani PL, Pavone E, Storti S, Moretti L, Fattori PP, Guardigni L, Falini B, Gobbi M, Gentilini P, Lauta VM, Bendandi M, Gherlinzoni F, Magagnoli M, Venturi S, Aitini E, Tabanelli M, Leone G, Liso V, Tura S (1997) Randomized trial with or without granulocyte colony-stimulating factor as adjunct to induction VNCOP-B treatment of elderly high-grade non-Hodgkin's lymphoma. Blood 89(11):3974–3979
Scott SD, Chrischilles EA, Link BK, Delgado DJ, Fridman M, Stolshek BS (2003) Days of prophylactic filgrastim use to reduce febrile neutropenia in patients with non-Hodgkin's lymphoma treated with chemotherapy. J Manag Care Pharm 9(2 Suppl):15–21
Jacobson JO, Grossbard M, Shulman LN, Neuberg D (2000) CHOP chemotherapy with preemptive granulocyte colony-stimulating factor in elderly patients with aggressive non-Hodgkin's lymphoma: a dose-intensity analysis. Clin Lymphoma 1(3):211–217, discussion 218
Peters FP, Fickers MM, Erdkamp FL, Wals J, Wils JA, Schouten HC (2001) The effect of optimal treatment on elderly patients with aggressive non-Hodgkin's lymphoma: more patients treated with unaffected response rates. Ann Hematol 80(7):406–410
Pfreundschuh M, Trumper L, Kloess M, Schmits R, Feller AC, Rube C, Rudolph C, Reiser M, Hossfeld DK, Eimermacher H, Hasenclever D, Schmitz N, Loeffler M (2004) Two-weekly or 3-weekly CHOP chemotherapy with or without etoposide for the treatment of elderly patients with aggressive lymphomas: results of the NHL-B2 trial of the DSHNHL. Blood 104(3):634–641. doi:10.1182/blood-2003-06-2095
National Comprehensive Cancer Network (2011) NCCN Clinical Practice Guidelines in Oncology: Myeloid Growth Factors, version 1.2011. National Comprehensive Cancer Network, Fort Washington, PA
Smith TJ, Khatcheressian J, Lyman GH, Ozer H, Armitage JO, Balducci L, Bennett CL, Cantor SB, Crawford J, Cross SJ, Demetri G, Desch CE, Pizzo PA, Schiffer CA, Schwartzberg L, Somerfield MR, Somlo G, Wade JC, Wade JL, Winn RJ, Wozniak AJ, Wolff AC (2006) 2006 update of recommendations for the use of white blood cell growth factors: an evidence-based clinical practice guideline. J Clin Oncol 24(19):3187–3205. doi:10.1200/JCO.2006.06.4451
Lyman GH, Lyman CH, Agboola O (2005) Risk models for predicting chemotherapy-induced neutropenia. Oncologist 10(6):427–437. doi:10.1634/theoncologist.10-6-427
Pettengell R, Bosly A, Szucs TD, Jackisch C, Leonard R, Paridaens R, Constenla M, Schwenkglenks M (2009) Multivariate analysis of febrile neutropenia occurrence in patients with non-Hodgkin lymphoma: data from the INC-EU Prospective Observational European Neutropenia Study. Br J Haematol 144(5):677–685. doi:10.1111/j.1365-2141.2008.07514.x
von Minckwitz G, Schwenkglenks M, Skacel T, Lyman GH, Pousa AL, Bacon P, Easton V, Aapro MS (2009) Febrile neutropenia and related complications in breast cancer patients receiving pegfilgrastim primary prophylaxis versus current practice neutropaenia management: results from an integrated analysis. Eur J Cancer 45(4):608–617. doi:10.1016/j.ejca.2008.11.021
Crawford J (2006) Risk assessment and guidelines for first-cycle colony-stimulating factor use in the management of chemotherapy-induced neutropenia. Oncology (Williston Park) 20(5 Suppl 4):22–28
Crawford J, Glaspy JA, Stoller RG, Tomita DK, Vincent ME, McGuire BW, Ozer H (2005) Final results of a placebo-controlled study of filgrastim in small-cell lung cancer: exploration of risk factors for febrile neutropenia. Support Cancer Ther 3(1):36–46. doi:10.3816/SCT.2005.n.023
Lyman GH, Dale DC, Friedberg J, Crawford J, Fisher RI (2004) Incidence and predictors of low chemotherapy dose-intensity in aggressive non-Hodgkin's lymphoma: a nationwide study. J Clin Oncol 22(21):4302–4311. doi:10.1200/JCO.2004.03.213
Morrison VA, Picozzi V, Scott S, Pohlman B, Dickman E, Lee M, Lawless G, Kerr R, Caggiano V, Delgado D, Fridman M, Ford J, Carter WB, Oncology Practice Pattern Study Working Group (2001) The impact of age on delivered dose intensity and hospitalizations for febrile neutropenia in patients with intermediate-grade non-Hodgkin's lymphoma receiving initial CHOP chemotherapy: a risk factor analysis. Clin Lymphoma 2(1):47–56
Balducci L, Al-Halawani H, Charu V, Tam J, Shahin S, Dreiling L, Ershler WB (2007) Elderly cancer patients receiving chemotherapy benefit from first-cycle pegfilgrastim. Oncologist 12(12):1416–1424. doi:10.1634/theoncologist.12-12-1416
Crawford J, Ozer H, Stoller R, Johnson D, Lyman G, Tabbara I, Kris M, Grous J, Picozzi V, Rausch G et al (1991) Reduction by granulocyte colony-stimulating factor of fever and neutropenia induced by chemotherapy in patients with small-cell lung cancer. N Engl J Med 325(3):164–170. doi:10.1056/NEJM199107183250305
Green MD, Koelbl H, Baselga J, Galid A, Guillem V, Gascon P, Siena S, Lalisang RI, Samonigg H, Clemens MR, Zani V, Liang BC, Renwick J, Piccart MJ (2003) A randomized double-blind multicenter phase III study of fixed-dose single-administration pegfilgrastim versus daily filgrastim in patients receiving myelosuppressive chemotherapy. Ann Oncol 14(1):29–35
Holmes FA, O'Shaughnessy JA, Vukelja S, Jones SE, Shogan J, Savin M, Glaspy J, Moore M, Meza L, Wiznitzer I, Neumann TA, Hill LR, Liang BC (2002) Blinded, randomized, multicenter study to evaluate single administration pegfilgrastim once per cycle versus daily filgrastim as an adjunct to chemotherapy in patients with high-risk stage II or stage III/IV breast cancer. J Clin Oncol 20(3):727–731
Trillet-Lenoir V, Green J, Manegold C, Von Pawel J, Gatzemeier U, Lebeau B, Depierre A, Johnson P, Decoster G, Tomita D et al (1993) Recombinant granulocyte colony stimulating factor reduces the infectious complications of cytotoxic chemotherapy. Eur J Cancer 29A(3):319–324
Hillestad R, Bigelow J, Bower A, Girosi F, Meili R, Scoville R, Taylor R (2005) Can electronic medical record systems transform health care? Potential health benefits, savings, and costs. Health Aff (Millwood) 24(5):1103–1117. doi:10.1377/hlthaff.24.5.1103
Acknowledgments
The authors thank Sharon Hunter, Sejal Badre, and Wayne Sheldon for the biostatistical support. Kerri Hebard-Massey (Amgen Inc.) provided medical writing support.
Conflict of interest
This study was sponsored by Amgen Inc. SW is an employee of and stockholder in Amgen Inc. EA was an employee of Amgen Inc. at the time the study was performed. LS has a consultancy or advisory role with Amgen Inc. MS has no conflicts to declare. Authors had full control of the primary data and agree to allow the journal to review the data if requested.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Schwartzberg, L.S., Saleh, M., Whittaker, S. et al. Severe neutropenia and relative dose intensity among patients <65 and ≥65 years with non-Hodgkin’s lymphoma receiving CHOP-based chemotherapy. Support Care Cancer 22, 1833–1841 (2014). https://doi.org/10.1007/s00520-014-2157-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00520-014-2157-8