Abstract
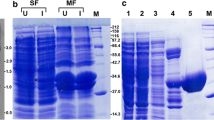
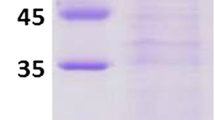
The catalysis of malate dehydrogenase (MDH) in Plasmodium falciparum (pfMDH) which involves NAD/NADH coupling is crucial for the parasite’s pathogenicity. Primers were designed based on the P. falciparum genome resource, and these facilitated the cloning of a gene coding for pfMDH from a local clinical isolate. The DNA sequence of the cloned gene revealed an open-reading frame that encodes a protein of 313 amino acids. After induction in Escherichia coli BL21, enzyme assays of the expressed pfMDH purified by affinity chromatography exhibited significant enzyme activity of about 50 U/mg, where one unit (U) of enzyme activity is defined as the amount of enzyme oxidising 1 μol NADH/min. Based on its phylogenetic status amongst MDHs and lactate dehydrogenases (LDHs), the cloned gene was clearly defined as belonging to the NADH-dependent [LDH-like] MDHs. It is noteworthy that pfMDH harbours unique structural characteristics potentially useful for screening drugs specific for disabling parasitic enzymes.



Similar content being viewed by others
References
Bzik DJ, Fox BA, Gonyer K (1993) Expression of Plasmodium falciparum lactate dehydrogenase in Escherichia coli. Mol Biochem Parasitol 59:155–166
Certa U, Ghersa P, Dobeli H, Matile H, Kocher HP (1988) Aldolase activity of a Plasmodium falciparum protein with protective properties. Science 240:1036–1038
Chapman ADM, Cortes A, Dafforn TR, Clarke AR, Brady RL (1999) Structural basis of substrate specificity in malate dehydrogenases: crystal structure of a ternary complex of porcine cytoplasmic malate dehydrogenase, α-ketomalonate and tetrahydoNAD. J Mol Biol 285:703–712
Florens L, Washburn MP, Raine JD, Anthony RM, Graingerk M, Haynes JD, Moch JK, Muster N, Sacci JB, Tabb DL, Witney AA, Wolters D, Wu Y, Gardner MJ, Holderk AA, Sinden RE, Yates JR, Carucci DJ (2002) A proteomic view of the Plasmodium falciparum life cycle. Nature 419(3):520–526
Gardner MJ, Hall N, Fung E, White O, Berriman M, Hyman RW, Carlton JM, Pain A, Nelson KE, Bowman S, Paulsen IT, James K, Eisen JA, Rutherford K, Salzberg SL, Craig A, Kyes S, Chan MS, Nene V, Shallom SJ, Suh B, Peterson J, Angiuoli S, Pertea M, Allen J, Selengut J, Haft D, Mather MW, Vaidya AB, Martin DM, Fairlamb AH, Fraunholz MJ, Roos DS, Ralph SA, McFadden GI, Cummings LM, Subramanian GM, Mungall C, Venter JC, Carucci DJ, Hoffman SL, Newbold C, Davis RW, Fraser CM, Barrell B (2002) Genome sequence of the human malaria parasite Plasmodium falciparum. Nature 419(6):498–511
Gietl C (1992) Malate dehydrogenase isoenzymes: cellular locations and role in the flow of metabolites between the cytoplasm and cell organelles. Biochim Biophys Acta 1100:217–34
Goward RC, Nicholls DJ (1994) Malate dehydrogenase: a model for structure, evolution and catalysis. Prot Sci 3:1883–1888
Hicks KE, Read M, Holloway SP, Sims PFG, Hyde JE (1991) Glycolytic pathway of the human malaria parasite Plasmodium falciparum: primary sequence analysis of the gene encoding 3-phosphoglycerate kinase and chromosomal mapping studies. Gene 100:123–129
Kaslow DC, Hill S (1990) Cloning metabolic pathway genes by complementation in Escherichia coli: isolation and expression of Plasmodium falciparum glucose phosphate isomerase. J Biol Chem 265:12337–12341
Lang-Unnasch N (1992) Purification and properties of Plasmodium falciparum malate dehydrogenase. Mol Biochem Parasitol 50:17–25
Madern D (2002) Molecular evolution within the l -malate and l -lactate dehydrogenase superfamily. J Mol Evol 54:825–840
Sim J, Chin HS, Wong E, Sim TS (in press) Conserved structural modules and bonding networks in isopenicillin N synthase related non-haem iron-dependent oxygenase and oxidases. J Mol Catal
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Chan, M., Sim, T.S. Functional characterization of an alternative [lactate dehydrogenase-like] malate dehydrogenase in Plasmodium falciparum . Parasitol Res 92, 43–47 (2004). https://doi.org/10.1007/s00436-003-0996-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00436-003-0996-1