Abstract
Purpose
Recent studies have reported that margins alone do not predict survival in patients with a positive chemotherapy response. The aim of this retrospective study is to analyze the surgical and oncological outcomes of patients who underwent chemotherapy and liver resection for colorectal liver metastases (CRLM) with lesions detached from the main hepatic veins, comparing the vein-preserving (VP) approach with traditional surgery.
Methods
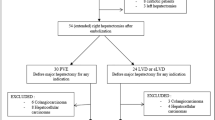
Fourteen patients undergoing VP surgery from January 2006 to January 2013 were matched in a 1:2 ratio with a control group (CG) of 28 patients undergoing traditional resection.
Results
The median follow-up was 43 months. The radiological response was classified as ‘partial response’ in eight VP patients and 11 controls (57 vs. 39 %, p = 0.249) and as ‘stable disease’ in three VP patients and 9 controls (21 vs. 32 %, p = 0.465). Ten VP (71.4 %) and twenty CG patients (71.4 %) experienced tumor relapse (p = 0.99). No venous edge recurrences were recorded in the VP group, whereas 1/13 (7.7 %) was observed in the control group (p = 0.99). The pathological response rate was 64 vs. 39 % (p = 0.037) in VP and CG patients, respectively. The 5-year recurrence-free survival rate was 24 % for VP patients and 25 % for CG patients (p = 0.431).
Conclusion
In patients with a positive CT response, CRLM can be detached from the hepatic veins, as the oncological outcome is similar to that of a larger resection. The VP approach offers the possibility to enlarge the surgical indications, thus optimizing future surgical treatment chances.



Similar content being viewed by others
References
Karsa LV, Lignini TA, Patnick J, Lambert R, Sauvaget C (2010) The dimensions of the CRC problem. Best Pract Res Cl Ga 24(4):381–396
Legolvan MP, Resnick M (2010) Pathobiology of colorectal cancer hepatic metastases with an emphasis on prognostic factors. J Sur Oncol 102(8):898–908
Scheele J, Stangl R, Altendorf-Hofmann A, Gall FP (1991) Indicators of prognosis after hepatic resection for colorectal secondaries. Surgery 110(1):13–29
Allen PJ, Kemeny N, Jarnagin W, DeMatteo R, Blumgart L, Fong Y (2003) Importance of response to neoadjuvant chemotherapy in patients undergoing resection of synchronous colorectal liver metastases. J Gastrointest Surg 7:109–15
Nordlinger B, Sorbye H, Glimelius B et al (2008) Perioperative chemotherapy with FOLFOX4 and surgery versus surgery alone for resectable liver metastases from colorectal cancer (EORTC Intergroup trial 40983): a randomised controlled trial. Lancet 371:1007–16
Parks R, Gonen M, Kemeny N et al (2007) Adjuvant chemotherapy improves survival after resection of hepatic colorectal metastases: analysis of data from two continents. J Am Coll Surg 204:753–61
Nordlinger B, Guiguet M, Vaillant JC et al (1996) Surgical resection of colorectal carcinoma metastases to the liver: a prognostic scoring system to improve case selection, based on 1568 patients. Cancer 77:1254–62
Portier G, Elias D, Bouche O et al (2006) Multicenter randomized trial of adjuvant fluorouracil and folinic acid compared with surgery alone after resection of colorectal liver metastases: FFCD ACHBTH AURC 9002 trial. J Clin Oncol 24:4976–82
Mitry E, Fields AL, Bleiberg H et al (2008) Adjuvant chemotherapy after potentially curative resection of metastases from colorectal cancer: a pooled analysis of two randomized trials. J Clin Oncol 26:4906–11
Gruenberger T, Arnold D, Rubbia-Brandt L (2012) Pathologic response to bevacizumab-containing chemotherapy in patients with colorectal liver metastases and its correlation with survival. Surg Oncol 21(4):309–15
Pawlik TM, Schulick RD, Choti MA (2008) Expanding criteria for resectability of colorectal liver metastases. Oncologist 13(1):51–64
Busquets J, Pelaez N, Alonso S et al (2006) The study of cavitational ultrasonically aspirated material during surgery for colorectal liver metastases as a new concept in resection margin. Ann Surg 244:634–5
Altendorf-Hofmann A, Scheele J (2003) A critical review of the major indicators of prognosis after resection of hepatic metastases from colorectal carcinoma. Surg Oncol Clin N Am 12:165–92
Figueras J, Burdio F, Ramos E et al (2007) Effect of subcentimeter nonpositive resection margin on hepatic recurrence in patients undergoing hepatectomy for colorectal liver metastases. Evidences from 663 liver resections. Ann Oncol 18:1190–95
Are C, Gonen M, Zazzali K et al (2007) The impact of margins on outcome after hepatic resection for colorectal metastasis. Ann Surg 246:295–300
Pawlik TM, Scoggins CR, Zorzi D et al (2005) Effect of surgical margin status on survival and site of recurrence after hepatic resection for colorectal metastases. Ann Surg 241:715–24
Nuzzo G, Giuliante F, Ardito F et al (2008) Influence of surgical margin on type of recurrence after liver resection for colorectal metastases: a single-center experience. Surgery 143:384–93
Ayez N, Lalmahomed ZS, Eggermont AM, Ijzermans JN, de Jonge J, van Montfort K, Verhoef C (2012) Outcome of microscopic incomplete resection (R1) of colorectal liver metastases in the era of neoadjuvant chemotherapy. Ann Surg Oncol 19(5):1618–27
de Haas RJ, Wicherts DA, Flores E et al (2008) R1 resection by necessity for colorectal liver metastases: is it still a contraindication to surgery? Ann Surg 248:626–637
Hamady ZZ, Lodge JP, Welsh FK et al (2014) One-millimeter cancer-free margin is curative for colorectal liver metastases: a propensity score case-match approach. Ann Surg 259(3):543–8
Andreou A, Aloia TA, Brouquet A, Dickson PV, Zimmitti G, Maru DM, Kopetz S, Loyer EM, Curley SA, Abdalla EK, Vauthey JN (2013) Margin status remains an important determinant of survival after surgical resection of colorectal liver metastases in the era of modern chemotherapy. Ann Surg 257(6):1079–88
Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, Dancey J, Arbuck S, Gwyther S, Mooney M, Rubinstein L, Shankar L, Dodd L, Kaplan R, Lacombe D, Verweij J (2009) New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer 45(2):228–47
Strasberg SM (2005) Nomenclature of hepatic anatomy and resections: a review of the Brisbane 2000 system. J Hepatobiliary Pancreat Surg 12:351–55
Slankamenac K, Graf R, Barkun J, Puhan MA, Clavien PA (2013) The comprehensive complication index: a novel continuous scale to measure surgical morbidity. Ann Surg 258(1):1–7
Fong Y, Fortner J, Sun RL, Brennan MF, Blumgart LH (1999) Clinical score for predicting recurrence after hepatic resection for metastatic colorectal cancer: analysis of 1001 consecutive cases. Ann Surg 230(3):309–18, discussion 318–21
Charnsangavej C, Clary B, Fong Y et al (2006) Selection of patients for resection of hepatic colorectal metastases: expert consensus statement. Ann Surg Oncol 13:1261–68
Gold JS, Are C, Kornprat P et al (2008) Increased used of parenchymal-sparing surgery for bilateral liver metastases from colorectal cancer is associated with improved mortality without change in oncologic outcome. Trends in treatment over time in 440 patients. Ann Surg 247:109–17
Muratore A, Ribeiro D, Zimmitti G et al (2010) Resection margin and recurrence-free survival after liver resection of colorectal metastases. Ann Surg Oncol 17:1324–29
Brudvik KW, Bains SJ, Seeberg LT, Labori KJ, Waage A, Taskén K, Aandahl EM, Bjørnbeth BA (2013) Aggressive treatment of patients with metastatic colorectal cancer increases survival: a scandinavian single-center experience. HPB Surg 2013:727095
Primrose JN (2010) Surgery for colorectal liver metastases. Br J Cancer 102(9):1313–8
Pinson CW, Wright JK, Chapman WC, Garrard CL, Blair TK, Sawyers JL (1996) Repeat hepatic surgery for colorectal cancer metastasis to the liver. Ann Surg 223(6):765–76
Adam R, Pascal G, Azoulay D, Tanaka K, Castaing D, Bismuth H (2003) Liver resection for colorectal metastases: the third hepatectomy. Ann Surg 238(6):871–84
Wicherts DA, Miller R, de Haas RJ, Bitsakou G, Vibert E, Veilhan LA, Azoulay D, Bismuth H, Castaing D, Adam R (2008) Long-term results of two-stage hepatectomy for irresectable colorectal cancer liver metastases. Ann Surg 248(6):994–1005
de Santibañes E, Clavien PA (2012) Playing Play-Doh to prevent postoperative liver failure: the "ALPPS" approach. Ann Surg 255(3):415–7
Hemming AW, Reed AI, Langham MR, Fujita S, van der Werf WJ, Howard RJ (2002) Hepatic vein reconstruction for resection of hepatic tumors. Ann Surg 235(6):850–8
Schnitzbauer AA, Lang SA, Goessmann H, Nadalin S, Baumgart J, Farkas SA et al (2012) Right portal vein ligation combined with in situ splitting induces rapid left lateral liver lobe hypertrophy enabling 2-staged extended right hepatic resection in small-for-size settings. Ann Surg 255:405–14
de Jong MC, Pulitano C, Ribero D, Strub J, Mentha G, Schulick RD, Choti MA, Aldrighetti L, Capussotti L, Pawlik TM (2009) Rates and patterns of recurrence following curative intent surgery for colorectal liver metastasis: an international multi-institutional analysis of 1669 patients. Ann Surg 250(3):440–8
Nakajima Y, Nagao M, Ko S et al (2001) Clinical predictors of recurrence site after hepatectomy for metastatic colorectal cancer. Hepatogastroenterology 48:1680–84
Tanaka K, Shimada H, Matsuo K, Ueda M, Endo I, Togo S (2007) Regeneration after two-stage hepatectomy vs. repeat resection for colorectal metastasis recurrence. J Gastrointest Surg 11(9):1154–61
Wakabayashi G et al (2015) Recommendations for laparoscopic liver resection: a report from the second international consensus conference held in Morioka. Ann Surg 261(4):619–29
Nordlinger B, Sorbye H, Glimelius B, Poston GJ, Schlag PM, Rougier P, Bechstein WO, Primrose JN, Walpole ET, Finch-Jones M, Jaeck D, Mirza D, Parks RW, Mauer M, Tanis E, Van Cutsem E, Scheithauer W, Gruenberger T (2013) Perioperative FOLFOX4 chemotherapy and surgery versus surgery alone for resectable liver metastases from colorectal cancer (EORTC 40983): long-term results of a randomised, controlled, phase 3 trial. Lancet Oncol 14(12):1208–15
Rees M, Tekkis PP, Welsh FK, O’Rourke T, John TG (2008) Evaluation of long-term survival after hepatic resection for metastatic colorectal cancer: a multifactorial model of 929 patients. Ann Surg 247(1):125–35
Tsai MS, Su YH, Ho MC, Liang JT, Chen TP, Lai HS, Lee PH (2007) Clinicopathological features and prognosis in resectable synchronous and metachronous colorectal liver metastasis. Ann Surg Oncol 14(2):786–94
Tsukamoto S, Kinugasa Y, Yamaguchi T, Shiomi A (2014) Survival after resection of liver and lung colorectal metastases in the era of modern multidisciplinary therapy. Int J Colorectal Dis 29(1):81–7
Brouquet A, Zimmitti G, Kopetz S, Stift J, Julié C, Lemaistre AI, Agarwal A, Patel V, Benoist S, Nordlinger B, Gandini A, Rivoire M, Stremitzer S, Gruenberger T, Vauthey JN, Maru DM (2013) Multicenter validation study of pathologic response and tumor thickness at the tumor-normal liver interface as independent predictors of disease-free survival after preoperative chemotherapy and surgery for colorectal liver metastases. Cancer 119(15):2778–88
Li Chang HH, Leeper WL, Chan G, Quan D, Driman DK (2012) Infarct-like necrosis: a distinct form of necrosis seen in colorectal carcinoma liver metastases treated with perioperative chemotherapy. Am J Surg Pathol 36:570–76
Korita PV, Wakai T, Shirai Y et al (2007) Intrahepatic lymphatic invasion independently predicts poor survival and recurrences after hepatectomy in patients with colorectal carcinoma liver metastases. Ann Surg Oncol 14:3472–80
Wakai T, Shirai Y, Sakata J, Valera VA, Korita PV, Akazawa K, Ajioka Y, Hatakeyama K (2008) Appraisal of 1 cm hepatectomy margins for intrahepatic micrometastases in patients with colorectal carcinoma liver metastasis. Ann Surg Oncol 15(9):2472–81
Kokudo N, Miki Y, Sugai S et al (2002) Genetic and histological assessment of surgical margins in resected liver metastases from colorectal carcinoma: minimum surgical margins for successful resection. Arch Surg 137:833–40
Hammond J, Muirhead W, Zaitoun AM, Cameron IC, Lobo DN (2012) Comparison of liver parenchymal ablation and tissue necrosis in a cadaveric bovine model using the Harmonic Scalpel, the LigaSure, the Cavitron Ultrasonic Surgical Aspirator and the Aquamantys devices. HPB (Oxford) 14(12):828–32
Acknowledgments
None.
Conflicts of interest
None.
Authors’ contributions
R. I. Troisi conceived of the study and its design. I. Bonadio, F. Tomassini, P. Smeets, L. Ferdinande, and L. J. Libbrecht participated in the acquisition of data. R. I. Troisi, I. Bonadio, F. Tomassini, G. Berardi, and K. De Paepe participated in the quality control of the data and algorithms. I. Bonadio, F. Tomassini, G. Berardi, and K. De Paepe participated in the statistical analysis. R. I. Troisi, I. Bonadio, F. Tomassini, G. Berardi, and K. De Paepe participated in the data analysis and interpretation. R. I. Troisi, I. Bonadio, F. Tomassini, and G. Berardi participated in the manuscript preparation. R. I. Troisi, I. Bonadio, F. Tomassini, and G. Berardi participated in the manuscript editing. R. I. Troisi, I. Bonadio, F. Tomassini, G. Berardi, L. Ferdinande, L. J. Libbrecht, Geboes, and S. Laurent participated in the manuscript review.
Permissions
This study is based on the data coming from a prospective kept database approved by the local IRB for retrospective analyses with the specific authorization number: B670200666
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Tomassini, F., Bonadio, I., Smeets, P. et al. Safety analysis of the oncological outcome after vein-preserving surgery for colorectal liver metastases detached from the main hepatic veins. Langenbecks Arch Surg 400, 683–691 (2015). https://doi.org/10.1007/s00423-015-1332-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00423-015-1332-9