Abstract
Purpose
The present review summarizes key papers on the elimination of endotoxin in human.
Results
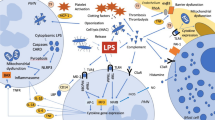
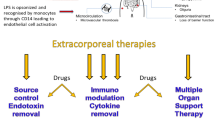
Lipopolysaccharides (LPS) are extremely strong stimulators of inflammatory reactions, act at very low concentrations, and are involved in the pathogenesis of sepsis and septic shock. Elimination of LPS is vital; therefore, therapeutic detoxification of LPS may offer new perspectives. Multiple mechanisms eliminate LPS in human comprising molecules that bind LPS and prevent it from signaling, enzymes that degrade and detoxify LPS, processes that inactivate LPS following uptake into the reticulo-endothelial system, and mechanisms of adaptation that modify target cells responding to LPS. These mechanisms are powerful and detoxification capacity adapts as required. Results of therapeutic interventions aiming at the removal of LPS by medication (immunoglobulins) or extracorporeal means are controversial. At least in part, animal experiments revealed increased survival. Human trials confirmed the positive effects on parameters of secondary importance, but not on morbidity or survival which was attributed to the heterogeneity of patients suffering from consequences of severe infectious diseases and sepsis.
Conclusion
The hypothesis of LPS-driven inflammatory processes remains very attractive. However, few therapeutic yet immature options have been developed to date.
Similar content being viewed by others
References
Greisman SE, Hornick RB (1969) Comparative pyrogenic reactivity of rabbit and man to bacterial endotoxin. Proc Soc Exp Biol Med 131:1154–1158
Bentala H, Verweij WR, Huizinga-Van der Vlag A, van Loenen-Weemaes AM, Meijer DK, Poelstra K (2002) Removal of phosphate from lipid A as a strategy to detoxify lipopolysaccharide. Shock 18:561–566
Buttenschoen K, Fathimani K, Carli Buttenschoen D (2010) Effect of major abdominal surgery on the host immune response to infection. Curr Opin Infect Dis 23:259–267
Munford RS (2005) Detoxifying endotoxin: time, place and person. J Endotoxin Res 11:69–84
Fischer MB, Prodeus AP, Nicholson-Weller A, Ma M, Murrow J, Reid RR, Warren HB, Lage AL, Moore FD Jr, Rosen FS, Carroll MC (1997) Increased susceptibility to endotoxin shock in complement C3- and C4-deficient mice is corrected by C1 inhibitor replacement. J Immunol 159:976–982
Buttenschoen K, Berger D, Strecker W, Carli Buttenschoen D, Stenzel K, Pieper T, Beger HG (2000) Association of endotoxemia and production of antibodies against endotoxins following multiple injuries. J Trauma 48:918–923
Gazzano-Santoro H, Meszaros K, Birr C, Carroll SF, Theofan G, Horwitz AH, Lim E, Aberle S, Kasler H, Parent JB (1994) Competition between rBPI23, a recombinant fragment of bactericidal/permeability-increasing protein, and lipopolysaccharide (LPS)-binding protein for binding to LPS and Gram-negative bacteria. Infect Immun 62:1185–1191
von der Mohlen MA, van Deventer SJ, Levi M, van den Ende B, Wedel NI, Nelson BJ, Friedmann N, Ten Cate JW (1995) Inhibition of endotoxin-induced activation of the coagulation and fibrinolytic pathways using a recombinant endotoxin-binding protein (rBPI23). Blood 85:3437–3443
Vesy CJ, Kitchens RL, Wolfbauer G, Albers JJ, Munford RS (2000) Lipopolysaccharide-binding protein and phospholipid transfer protein release lipopolysaccharides from Gram-negative bacterial membranes. Infect Immun 68:2410–2417
Domingues MM, Castanho MA, Santos NC (2009) rBPI(21) promotes lipopolysaccharide aggregation and exerts its antimicrobial effects by (hemi)fusion of PG-containing membranes. PLoS ONE 4:e8385
Golenbock DT, Hampton RY, Qureshi N, Takayama K, Raetz CR (1991) Lipid A-like molecules that antagonize the effects of endotoxins on human monocytes. J Biol Chem 266:19490–19498
Weinrauch Y, Katz SS, Munford RS, Elsbach P, Weiss J (1999) Deacylation of purified lipopolysaccharides by cellular and extracellular components of a sterile rabbit peritoneal inflammatory exudate. Infect Immun 67:3376–3382
Feulner JA, Lu M, Shelton JM, Zhang M, Richardson JA, Munford RS (2004) Identification of acyloxyacyl hydrolase, a lipopolysaccharide-detoxifying enzyme, in the murine urinary tract. Infect Immun 72:3171–3178
Verweij WR, Bentala H, Huizinga-Van der Vlag A, Miek vL-W, Kooi K, Meijer DK, Poelstra K (2004) Protection against an Escherichia coli-induced sepsis by alkaline phosphatase in mice. Shock 22:174–179
Bol-Schoenmakers M, Fiechter D, Raaben W, Hassing I, Bleumink R, Kruijswijk D, Maijoor K, Tersteeg-Zijderveld M, Brands R, Pieters R (2010) Intestinal alkaline phosphatase contributes to the reduction of severe intestinal epithelial damage. Eur J Pharmacol 633:71–77
Ge Y, Ezzell RM, Tompkins RG, Warren HS (1994) Cellular distribution of endotoxin after injection of chemically purified lipopolysaccharide differs from that after injection of live bacteria. J Infect Dis 169:95–104
Knolle PA, Gerken G (2000) Local control of the immune response in the liver. Immunol Rev 174:21–34
Ruiter DJ, van der Meulen J, Brouwer A, Hummel MJ, Mauw BJ, van der Ploeg JC, Wisse E (1981) Uptake by liver cells of endotoxin following its intravenous injection. Lab Invest 45:38–45
Jackson GD, Dai Y, Sewell WA (2000) Bile mediates intestinal pathology in endotoxemia in rats. Infect Immun 68:4714–4719
Jirillo E, Caccavo D, Magrone T, Piccigallo E, Amati L, Lembo A, Kalis C, Gumenscheimer M (2002) The role of the liver in the response to LPS: experimental and clinical findings. J Endotoxin Res 8:319–327
Satoh M, Ando S, Shinoda T, Yamazaki M (2008) Clearance of bacterial lipopolysaccharides and lipid A by the liver and the role of argininosuccinate synthase. Innate Immun 14:51–60
Vreugdenhil AC, Rousseau CH, Hartung T, Greve JW, van’t V, Buurman WA (2003) Lipopolysaccharide (LPS)-binding protein mediates LPS detoxification by chylomicrons. J Immunol 170:1399–1405
Levels JH, Abraham PR, van den Ende A, van Deventer SJ (2001) Distribution and kinetics of lipoprotein-bound endotoxin. Infect Immun 69:2821–2828
Diks SH, van Deventer SJ, Peppelenbosch MP (2001) Lipopolysaccharide recognition, internalisation, signalling and other cellular effects. J Endotoxin Res 7:335–348
Caron E, Hall A (1998) Identification of two distinct mechanisms of phagocytosis controlled by different Rho GTPases. Science 282:1717–1721
Underhill DM, Ozinsky A, Hajjar AM, Stevens A, Wilson CB, Bassetti M, Aderem A (1999) The Toll-like receptor 2 is recruited to macrophage phagosomes and discriminates between pathogens. Nature 401:811–815
Kitchens RL, Munford RS (1998) CD14-dependent internalization of bacterial lipopolysaccharide (LPS) is strongly influenced by LPS aggregation but not by cellular responses to LPS. J Immunol 160:1920–1928
Bunnell E, Lynn M, Habet K, Neumann A, Perdomo CA, Friedhoff LT, Rogers SL, Parrillo JE (2000) A lipid A analog, E5531, blocks the endotoxin response in human volunteers with experimental endotoxemia. Crit Care Med 28:2713–2720
Jaber BL, Pereira BJ (1997) Extracorporeal adsorbent-based strategies in sepsis. Am J Kidney Dis 30:S44–S56
Anspach FB (2001) Endotoxin removal by affinity sorbents. J Biochem Biophys Meth 49:665–681
Srimal S, Surolia N, Balasubramanian S, Surolia A (1996) Titration calorimetric studies to elucidate the specificity of the interactions of polymyxin B with lipopolysaccharides and lipid A. Biochem J 315(Pt 2):679–686
Kawatsu M, Wada J, Kitano M, Ogino E, Sakurai H, Furuyoshi S, Tani N (2006) Effects of a new extracorporeal system using CTR on mortality and inflammatory responses to bacterial toxin-induced multiple organ dysfunction syndrome in rabbits. Blood Purif 24:327–334
Taniguchi T, Hirai F, Takemoto Y, Tsuda K, Yamamoto K, Inaba H, Sakurai H, Furuyoshi S, Tani N (2006) A novel adsorbent of circulating bacterial toxins and cytokines: the effect of direct hemoperfusion with CTR column for the treatment of experimental endotoxemia. Crit Care Med 34:800–806
Bracht H, Hauser B, Ivanyi Z, Asfar P, Ehrmann U, Brueckner UB, Georgieff M, Radermacher P, Buttenschoen K (2009) Efficacy of an extracorporeal endotoxin adsorber system during hyperdynamic porcine endotoxemia. Eur Surg Res 43:53–60
Kojika M, Sato N, Yaegashi Y, Suzuki Y, Suzuki K, Nakae H, Endo S (2006) Endotoxin adsorption therapy for septic shock using polymyxin B-immobilized fibers (PMX): evaluation by high-sensitivity endotoxin assay and measurement of the cytokine production capacity. Ther Apher Dial 10:12–18
Ebihara I, Nakamura T, Shimada N, Shoji H, Koide H (1998) Effect of hemoperfusion with polymyxin B-immobilized fiber on plasma endothelin-1 and endothelin-1 mRNA in monocytes from patients with sepsis. Am J Kidney Dis 32:953–961
Blomquist S, Gustafsson V, Manolopoulos T, Pierre L (2009) Clinical experience with a novel endotoxin adsorbtion device in patients undergoing cardiac surgery. Perfusion 24:13–17
Ullrich H, Jakob W, Frohlich D, Rothe G, Prasser C, Drobnik W, Taeger K, Meier-Hellmann A, Reinhart K, Zimmermann M, Schmitz G (2001) A new endotoxin adsorber: first clinical application. Ther Apher 5:326–334
Reinhart K, Meier-Hellmann A, Beale R, Forst H, Boehm D, Willatts S, Rothe KF, Adolph M, Hoffmann JE, Boehme M, Bredle DL (2004) Open randomized phase II trial of an extracorporeal endotoxin adsorber in suspected Gram-negative sepsis. Crit Care Med 32:1662–1668
Buttenschoen K, Kornmann M, Berger D, Leder G, Beger HG, Vasilescu C (2008) Endotoxemia and endotoxin tolerance in patients with ARDS. Langenbecks Arch Surg 393:473–478
Nakamura T, Fujiwara N, Sato E, Kawagoe Y, Ueda Y, Yamada S, Koide H (2009) Effect of polymyxin B-immobilized fiber hemoperfusion on serum high mobility group box-1 protein levels and oxidative stress in patients with acute respiratory distress syndrome. ASAIO J 55:395–399
Nakamura T, Yamagishi SI (2010) PEDF and septic shock. Curr Mol Med 10:312–316
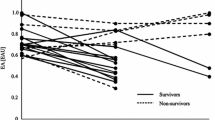
Marshall JC, Foster D, Vincent JL, Cook DJ, Cohen J, Dellinger RP, Opal S, Abraham E, Brett SJ, Smith T, Mehta S, Derzko A, Romaschin A (2004) Diagnostic and prognostic implications of endotoxemia in critical illness: results of the MEDIC Study. J Infect Dis 190:527–534
Yachamaneni S, Yushin G, Yeon SH, Gogotsi Y, Howell C, Sandeman S, Phillips G, Mikhalovsky S (2010) Mesoporous carbide-derived carbon for cytokine removal from blood plasma. Biomaterials 31:4789–4794
Bertok L (2005) Radio-detoxified endotoxin activates natural immunity: a review. Pathophysiology 12:85–95
Hurley JC, Levin J (1999) The relevance of endotoxin detection in sepsis. In: Brade H, Opal SM, Vogel SN, Morrison DC (eds) Endotoxin in health and disease. Marcel Dekker, New York, pp 841–854
Hurley JC (2003) Endotoxemia and Gram-negative bacteremia as predictors of outcome in sepsis: a meta-analysis using ROC curves. J Endotoxin Res 9:271–279
Acknowledgments
No funding was received for doing this overview. Klaus Buttenschoen, Peter Radermacher, and Hendrik Bracht are authors of the publication Eur Surg Res (2009) 43:53–60, “Efficacy of an Extracorporeal Endotoxin Adsorber System during Hyperdynamic Porcine Endotoxemia”. This study was supported by a research grant of Fresenius Company, Germany.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Buttenschoen, K., Radermacher, P. & Bracht, H. Endotoxin elimination in sepsis: physiology and therapeutic application. Langenbecks Arch Surg 395, 597–605 (2010). https://doi.org/10.1007/s00423-010-0658-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00423-010-0658-6