Abstract
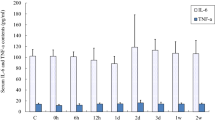
The purposes of this study were to investigate the effects of strenuous exercise on apoptosis of the gastrocnemius and soleus muscle fibers and clarify the role of oxidative metabolism in the strenuous exercise-induced apoptosis. The experiment was designed with 49 (n = 49) male, 24-week-old, L. Wistar albino rats. Strenuous exercise model was applied to 42 (n = 42) rats and seven (n = 7) rats served as rested controls. All rats were randomly assigned to one of the following groups (n = 7): rested control (C), immediately after exercise (0 h) and 3, 6, 12, 24, and 48 h after exercise. Apoptotic nuclei were shown by single stranded DNA (ssDNA) determination. Oxidative damage in mitochondrial fractions of the muscle tissues was evaluated by malondialdehyde (MDA) levels and reduced/oxidized glutathione (GSH/GSSG) ratios. Caspase-9, -8 and -3 activities and the level of cytochrome c (Cyt c) were measured in the cytosolic fractions of muscle tissues to follow mitochondrial-dependent (intrinsic) or ligand-mediated death receptor (extrinsic) pathways of apoptosis. Plasma interleukin-6 (IL-6) and tumor necrosis factor-α (TNF-α) levels were also determined. Based on our results, apoptosis is significantly triggered in muscle fibers by strenuous exercise (P < 0.05). Apoptosis in the soleus muscle tissues mostly depends on the intrinsic pathway and may be triggered by increased oxidative stress. In contrast, extrinsic pathway of apoptosis was predominant in the gastrocnemius muscle and increases of TNF-α and IL-6 may play a significant role.










Similar content being viewed by others
References
Armstrong R, Phelps R (1984) Muscle fiber type composition of rat hindlimb. Am J Anat 171:259-272
Bejma J, Ji LL (1999) Aging and acute exercise enhance free radical generation in rat skeletal muscle. J Appl Physiol 87:465-470
Buttke TM, Sandstrom PA (1994) Oxidative stress as a mediator of apoptosis. Immunol Today 15(1):7-10
Carraro U, Franceschi C (1997) Apoptosis of skeletal and cardiac muscles and physical exercise. Aging Clin Exp Res 9:19–34
Cereser C, Guichard J, Drai J, Bannier E, Garcia I, Parvaz P, Revol A (2001) Quantitation of reduced and total glutathione at the femtomole level by high-performance liquid chromatography with flourescence detection: application to red blood cells and cultured fibroblasts. J Chromatogr B 752:123–132
Cooper CE, Vollaard NBJ, Choueiri, Wilson MT (2002) Exercise, free radicals and oxidative stress. Biochem Soc Trans 30:280–285
Dalla LL, Sabbadini R, Renken C, Ravara B, Sandri M, Betto R, Angelini A, Vescovo G (2001) Apoptosis in the skeletal muscle of rats with heart failure is associated with increased serum levels of TNF-alpha and sphingosine. J Mol Cell Cardiol 33(10):1871–1878
Di Meo S, Venditti P (2001) Mitochondria in exercise-induced oxidative stress. Biol Signals Recept 10:125–140
Dirks AJ, Leeuwenburgh C (2006) Tumor necrosis factor a signaling in skeletal muscle: effects of age and caloric restriction. J Nutr Biochem 17:501–508
Duke RC, Ojcius DM, Young JD (1996) Cell suicide in health and disease. Sci Am 275:80–87
Fumarola C, Guidotti G (2004) Stress-induced apoptosis: toward symmetry with receptor-mediated cell death. Apoptosis 9:77–82
Garrido C, Galluzzi L, Brunet M, Puig PE, Didelot C, Kroemer G (2006) Mechanisms of cytochrome c release from mitochondria. Cell Death Differ 13:1423–1433
Goldenthal MJ, Garcia JM (2004) Mitochondrial signaling pathways: a receiver/integrator organelle. Mol Cell Biochem 262:1–16
Green DR, Reed JC (1998) Mitochondria and apoptosis. Science 281:1309–1312
Hermann C, Zeiher AM, Dimmeler S (1997) Shear stress inhibits H2O2-induced apoptosis of human endothelial cells by modulation of the glutathione redox cycle and nitric oxide synthase. Arterioscler Thromb Vasc Biol 17:3588–3592
Ji LL (1995) Exercise and oxidative stress: role of cellular antioxidant systems. In: Hollozy JO (ed) Exercise and sport sciences reviews. Williams & Wilkins, Baltimore, pp 135–139
Kadenbach B, Arnold S, Lee I, Huttemann M (2004) The possible role of cytochrome c oxidase in stress-induced apoptosis and degenerative diseases. Biochim Biophys Acta 1655:400–408
Kannan K, Jain SK (2000) Oxidative stress and apoptosis. Pathophysiology 7(27):153–163
Kroemer G (2003) Mitochondrial control of apoptosis: an introduction. Biochem Biophys Res Commun 304:433–435
Leeuwenburg C, Hansen PA, Hollosyz JO, Heinecke JW (1999) Hydoxyl radical generation during exercise increases mitochondrial protein oxidation and levels of urinary dityrosine. Free Radic Biol Med 27:186–192
Li H, Zhu H, Xu CJ, Yuan J (1998) Cleavage of BID by caspase-8 mediates the mitochondrial damage in the Fas pathway of apoptosis. Cell 94:491–501
Li JX, Tong CW, Xu DQ, Chan KM (1999) Changes in membrane fluidity and lipid peroxidation of skeletal muscle mitochondria after exhausting exercise in rats. Eur J Appl Physiol Occup Physiol 80:113–117
Liu OJ, Yeo H, Overvik-Douki E, Hagen T, Doniger SJ, Chyu DW, Brooks GA, Ames BN (2000) Chronically and acutely exercised rats: biomarkers of oxidative stress and endogenous antioxidants. J Appl Physiol 89:21–28
Lykkesfeldt J (2001) Determination of malondialdehyde as dithiobarbituric acid adducts in biological samples by HPLC with fluorescence detection: comparison with ultraviolent-visible spectrophotometry. Clin Chem 47:1725–1727
Markwell MAK, Hass SM, Bieber LL, Tolbert NE (1978) A modification of the Lowry procedure to simplify protein determination in the membrane and lipoprotein samples. Anal Chem 87:206–210
McArdle A, Pattwell D, Vasilaki A, Griffiths RD, Jacson MJ (2001) Contractile activity-induced oxidative stress: cellular origin and adaptive responses. Am J Physiol Cell Physiol 280:C621–C627
McArdle A, Van Der Meulen J, Close GL, Patwell D, Van Remmen H, Huang TT, Richardson AG, Epstein CJ, Faulkner JA, Jacson MJ (2004) The role of mitochondrial superoxide dismutase in contraction-induced generation of reactive oxygen species in skeletal muscle extracellular space. Am J Physiol Cell Physiol 286:C1152–C1158
McLennan HR, Degli Esposti M (2000) The contribution of mitochondrial respiratory complexes to the production of reactive oxygen species. J Bioenerg Biomembr 32:153–162
Meister A, Anderson ME (1983) Glutathione. Annu Rev Biochem 52:711–760
Nakagawa T, Zhu H, Morishima N, Li E, Xu J, Yankner BA, Yuan J (2000) Caspase-12 mediates endoplasmic-reticulum-specific apoptosis and cytotoxicity by amyloidbeta. Nature 403:98–103
Ostrowski K, Rohde T, Zacho M et al (1998) Evidence that interleukin-6 is produced in human skeletal muscle during prolonged running. J Physiol 508:949–953
Peake JM, Suzuki K, Wilson G, Hordern M, Yamaya K, Nosaka K, Mackinnon L, Coombes JS (2005a) Exercise-induced muscle damage, plasma cytokines and markers of neutrophil activation. Med Sci Sports Exerc 37:737–745
Peake JM, Suzuki K, Hordern M, Wilson G, Nosaka K, Coombes JS (2005b) Plasma cytokine changes in relation to exercise intensity and muscle damage. Eur J Appl Physiol 95(5-6):514–521
Pedersen BK, Hoffman-Goetz L (2000) Exercise and the immune system: regulation, integration and adaptation. Physiol Rev 80:1055–1081
Pedersen BK, Steensberg A, Schjerling P (2001) Exercise and interleukin-6. Curr Opin Hematol 8:137–141
Phaneuf S, Leewenburgh C (2001) Apoptosis and exercise. Med Sci Sports Exerc 33(3):393–396
Phaneuf S, Leeuwenburgh C (2002) Cytochrome c release from mitochondria in the aging heart: a possible mechanism for apoptosis with age. Am J Physiol Regul Integr Comp Physiol 282:R423–R430
Pistilli EE, Jackson JR, Alway SE (2006) Death receptor-associated pro-apoptotic signaling in aged skeletal muscle. Apoptosis 11:2115–2126
Podhorska-Okolow M, Sandri M, Zampieri S, Brun B, Rossini K, Carraro U (1998) Apoptosis of myofibers and satellite cells: exercise-induced damage in skeletal muscle of the mouse. Neuropath Appl Neurobiol 24:518–531
Podhorska-Okolow M, Krajewski B, Carraro U, Zabel M (1999) Apoptosis in mouse skeletal muscles after physical exercise. Folia Histochem Cytobiol 24:127–128
Pollack M, Leeuwenburgh C (2001) Apoptosis and aging: role of the mitochondria. J Gerontol A Biol Sci Med Sci 56(11):B475–B482
Powers SK, Criswell D, Lawler J, Ji LL, Martin D, Herb RA, Dudley G (1994) Influence of exercise and fiber type on antioxidant enzyme activity in rat skeletal muscle. Am J Physiol 266(35):R375–R380
Primeau AJ, Adhihetty PJ, Hood DA (2002) Apoptosis in heart and skeletal muscle. Can J Appl Physiol 27(4):349–395
Rajguru SU, Yeargans GS, Seidler NW (1994) Exercise causes oxidative damage to rat skeletal muscle microsomes while increasing cellular sulfhdryls. Life Sci 54:149–157
Reid MB, Li YP (2001) Cytokines and oxidative signaling in skeletal muscle. Acta Physiol Scand 171:225–232
Rossig L, Haendeler J, Hermann C, Malchow P, Urbich C, Zeiher AM, Dimmeler S (2000) Nitric oxide down-regulates MKP-3 mRNA levels: involvement in endothelial cell protection from apoptosis. J Biol Chem 275:25502–25507
Sandri M, Podhorska-Okolow M, Geromel V (1997) Exercise-induced myonuclear ubiquitination and apoptosis in dystrophin-deficient muscle of mice. J Neuropathol Exp Neurol 56:45-57
Sastre J, Pallardo VF, Vina J (2000) Mitochondrial oxidative stress plays a key role in aging and apoptosis. IUBMB Life 49:427–435
Scorrano L, Ashiya M, Buttle K, Weiler S, Oakes SA, Mannella CA, Korsmeyer SJ (2002) A distinct pathway remodels mitochondrial cristae and mobilizes cytochrome c during apoptosis. Dev Cell 2:55–67
Sharon P, Leeuwenburgh C (2001) Apoptosis and exercise. Med Sci Sports Exerc 33:393–396
Shephard RJ (2003) Adhesion molecules, catecholamines and leucocyte redistribution during and following exercise. Sports Med 33(4):261–284
Stevenson JR, Westermann J, Liebmann PM, Hortner M, Rinner I, Felsner P, Wolfler A, Schauenstein K (2001) Prolonged alphaadrenergic stimulation causes changes in leukocyte distribution and lymphocyte apoptosis in the rat. J Neuroimmunol 120:50–57
Stewart CE, Newcomb PV, Holly JM (2004) Multifaceted roles of TNF-alpha in myoblast destruction: a multitude of signal transduction pathways. J Cell Physiol 198:237–247
Sun XM, MacFarlane M, Zhuang J, Wolf BB, Green DR, Cohen GM (1999) Distinct caspase cascades are initiated in receptor-mediated and chemical-induced apoptosis. J Biol Chem 274:5053–5060
Suzuki A, Tsutomi Y, Shimizu M, Matsuzawa A (1999) Another cell death induction system: TNF-alpha acts as a ligand for Fas in vaginal cells. Cell Death Differ 6(7):638–643
Suzuki K, Yamada M, Kurakake S, Okamura N, Yamaya K, Liu O, Kudoh S, Kowatari K, Nakaji S, Sugawara K (2000) Circulating cytokines and hormones with immunosuppressive but neutrophil-priming potentials rise endurance exercise in humans. Eur J Appl Physiol 81:281–287
Van Gurp M, Festjens N, Van Loo G, Saelens X, Vandenabeele P (2003) Mitochondrial intermembrane proteins in cell death. Biochem Biophys Res Commun 304:487–497
Vasilaki A, Mansouri A, Remmen HV, van der Meulen JH, Larkin L, Richardson AG, McArdle A, Faulkner JA, Jackson MJ (2006) Free radical generation by skeletal muscle of adult and old mice: effect of contractile activity. Aging Cell 5:109–117
Willis TW, Dallman P, Brooks GA (1988) Physiological and biochemical correlates of increased work in trained iron-deficient rats. J Appl Physiol 65(1):256-263
Yuan J, Murrell GA, Trickett A, Wang MX (2003) Involvement of cytochrome c release and caspase-3 activation in the oxidative stress-induced apoptosis in human tendon fibroblasts. Biochim Biophys Acta 1641(1):35–41
Acknowledgments
This work was supported by Dokuz Eylul University Research Foundation Grant no: 036.01.01.05. The authors thank Dr. Memduh Bulbul for his excellent technical assistance on the HPLC analysis.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Koçtürk, S., Kayatekin, B.M., Resmi, H. et al. The apoptotic response to strenuous exercise of the gastrocnemius and solues muscle fibers in rats. Eur J Appl Physiol 102, 515–524 (2008). https://doi.org/10.1007/s00421-007-0612-7
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00421-007-0612-7