Abstract
Purpose
To evaluate the long-term safety and efficacy of intrastromal bevacizumab for treatment of deep corneal neovascularization in candidates for high-risk cornea grafting.
Methods
A single-center retrospective study involving 14 eyes of 14 patients with chronic deep corneal neovascularization, treated with intrastromal bevacizumab by a single provider from 2011 to present. Intrastromal bevacizumab (0.05–0.1 mL of 2.5 mg/0.1 mL) was administered every 4–8 weeks. On average 1–3 intrastromal injections were performed prior to corneal grafting (penetrating keratoplasty or deep anterior lamellar keratoplasty).
Results
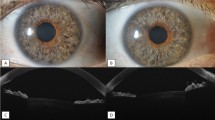
64.2% patients had neurotrophic keratitis secondary to herpes zoster or simplex. Neovascularization was encroaching the visual axis in 50% and was paracentral in 42.8%. After intrastromal bevacizumab injection, 14.2% had complete regression of neovascularization, avoiding the need of future corneal transplant. Persistent neovascularization was noticed in 21.4%. Successful penetrating keratoplasty was performed in 57% of patients. Minimal adverse effects were noted; temporary epithelial defect was seen in two eyes and self-limited intrastromal hemorrhage in one. There was no evidence of recurrence of neovascularization or graft rejection in the transplant group (mean follow-up 3 years).
Conclusion
Intrastromal bevacizumab appears to be a safe and effective modality in the treatment of chronic corneal neovascularization, producing durable regression of corneal neovascularization and allowing for durable success of subsequent corneal transplants in high-risk patients.





Similar content being viewed by others
References
George AJ, Larkin DF (2005) Corneal transplantation: the forgotten graft. Am J Transplant 4(5):678–685
Vassileva PI, Hergeldzhieva TG (2009) Avastin use in high risk corneal transplantation. Graefes Arch Clin Exp Ophthalmol 247(12):1701–1706
Dastjerdi MH, Al-arfaj KM, Nallasamy N et al (2009) Topical bevacizumab in the treatment of corneal neovascularization: results of a prospective, open-label, noncomparative study. Arch Ophthalmol 127(4):381–389
Chang JH, Garg NK, Lunde E, Han KY, Jain S, Azar DT (2012) Corneal neovascularization: an anti-VEGF therapy review. Surv Ophthalmol 57(5):415–429
Uy HS, Chan PS, Ang RE (2008) Topical bevacizumab and ocular surface neovascularization in patients with Stevens-Johnson syndrome. Cornea 27(1):70–73
Kim TI, Kim SW, Kim S et al (2008) Inhibition of experimental corneal neovascularization by using subconjunctival injection of bevacizumab (Avastin). Cornea 27(3):349–352
Cheng SF, Dastjerdi MH, Ferrari G et al (2012) Short-term topical bevacizumab in the treatment of stable corneal neovascularization. Am J Ophthalmol 154(6):940–948.e1
Yeung SN, Lichtinger A, Kim P, Amiran MD, Slomovic AR (2011) Combined use of subconjunctival and intracorneal bevacizumab injection for corneal neovascularization. Cornea 30(10):1110–1114
Vieira AC, Höfling-lima AL, Gomes JÁ, Dd F, Farah ME, Belfort R (2012) Intrastromal injection of bevacizumab in patients with corneal neovascularization. Arq Bras Oftalmol 75(4):277–279
Sarah B, Ibtissam H, Mohammed B, Hasna S, Abdeljalil M (2016) Intrastromal injection of bevacizumab in the management of corneal neovascularization: about 25 Eyes. J Ophthalmol
Lichtinger A, Yeung SN, Kim P, Amiran MD, Elbaz U, Slomovic AR (2014) Corneal endothelial safety following subconjunctival and intrastromal injection of bevacizumab for corneal neovascularization. Int Ophthalmol 34(3):597–601
Hashemian MN, Zare MA, Rahimi F, Mohomadpour M (2011) Deep intrastromal bevacizumab injection for management of corneal stromal vasccuzarization after deep anterior lamellar keratoplasty, a novel technique. Cornea 30(2):215–218
Gupta D, Illingworth C (2011) Treatments for corneal neovascularization: a review. Cornea 30(8):927–938
Mohammadpour M (2013) Deep intrastromal injection of bevacizumab for the management of corneal neovascularization. Cornea 32(1):109–110
Kim JH, Seo HW, Han HC, Lee JH, Choi SK, Lee D (2013) The effect of bevacizumab versus ranibizumab in the treatment of corneal neovascularization: a preliminary study. Korean J Ophthalmol 27(4):235–242
Ahn YJ, Hwang HB, Chung SK (2014) Ranibizumab injection for corneal neovascularization refractory to bevacizumab treatment. Korean J Ophthalmol 28(2):177–180
Kim YC, Grossniklaus HE, Edelhauser HF, Prausnitz MR (2014) Intrastromal delivery of bevacizumab using microneedles to treat corneal neovascularization. Invest Ophthalmol Vis Sci 55(11):7376–7386
Yoon HJ, Kim MK, Seo KY, Ueta M, Yoon KC (2019) Effectiveness of photodynamic therapy with verteporfin combined with intrastromal bevacizumab for corneal neovascularization in Stevens-Johnson syndrome. Int Ophthalmol 39(1):55–62
Yin J, Jacobs DS (2019) Long-term outcome of using Prosthetic Replacement of Ocular Surface Ecosystem (PROSE) as a drug delivery system for bevacizumab in the treatment of corneal neovascularization. Ocul Surf 17(1):134–141
Faraj LA, Said DG, Al-aqaba M, Otri AM, Dua HS (2016) Clinical evaluation and characterisation of corneal vascularisation. Br J Ophthalmol 100(3):315–322
Zakaria N, van Grasdorff S, Wouters K et al (2012) Human tears reveal insights into corneal neovascularization. PLoS One 7(5):e36451
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Gupta, A.A., Mammo, D.A. & Page, M.A. Intrastromal bevacizumab in the management of corneal neovascularization: a retrospective review. Graefes Arch Clin Exp Ophthalmol 258, 167–173 (2020). https://doi.org/10.1007/s00417-019-04519-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00417-019-04519-4