Abstract
Background
During retinal detachment, premature apoptosis of photoreceptors and a loss of optimally corrected visual acuity occur. We hypothesized that retinal cell death and generation of ceramide, a pro-apoptotic lipid, would progress as a function of time following experimental retinal detachment, and undertook to define the appropriate temporal window.
Methods
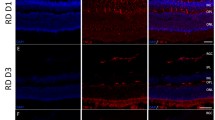
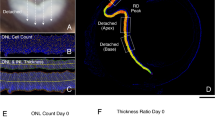
Unilateral retinal detachment was induced in white New Zealand rabbits by subretinal injection of sodium hyaluronate. In experimental animals, we injected sphingosine-1-P into the vitreous 2 hours before retinal detachment. Both eyes were removed on days 1, 3 and 6 for histological and biochemical examination. The number of photoreceptors was counted in section, the level of apoptosis was assessed using the TUNEL assay, and the production of ceramide was analyzed in situ with immunohistochemistry. The concentration of ceramide was also determined on retinal homogenates using a diacylglycerol kinase assay.
Results
We confirmed that the average number of live photoreceptors decreased gradually after retinal detachment. In eyes pre-treated with sphingosine-1-P the number of apoptotic photoreceptors was significantly lower. The proportion of apoptotic photoreceptors (14%) remained constant as a function of time in the window studied. As compared to controls, the detached retina showed intense ceramide immunostaining that was prominent in the photoreceptors, but also present to a lesser extent in other retinal layers. The total concentration of intra-retinal ceramide increased by 40% on the first day and continued augmenting through the sixth day after retinal detachment.
Conclusions
Retinal apoptosis during experimental retinal detachment is associated with in vivo production of ceramide.






Similar content being viewed by others
References
Chang CJ, Lai WW, Edward DP, Tso MO (1995) Apoptotic photoreceptor cell death after traumatic retinal detachment in humans. Arch Ophthalmol 113:880–886
Hisatomi T, Sakamoto T, Goto Y, Yamanaka I, Oshima Y, Ishibashi T, Inomata H, Susin SA, Kroemer G (2002) Critical role of photoreceptor apoptosis in functional damage after retinal detachment. Curr Eye Res 24:161–172, doi:10.1076/ceyr.24.3.161.8305
Burton TC (1982) Recovery of visual acuity after retinal detachment involving the macula. Trans Am Ophthalmol Soc 80:475–497
Creagh EM, Conroy H, Martin SJ (2003) Caspase-activation pathways in apoptosis and immunity. Immunol 193:10–21, doi:10.1034/j.1600-065X.2003.00048.x
Cuvillier O, Andrieu-Abadie N, Ségui B, Malagarie-Cazenave S, Tardy C, Bonhoure E, Levade T (2003) Voies de signalisation de l’apoptose médiées par les sphingolipides. J Soc Biol 197:217–221
Levade T, Malagarie-Cazenave S, Gouaze V, Segui B, Tardy C, Betito S, Andrieu-Abadie N, Cuvillier O (2002) Ceramide in apoptosis: a revisited role. Neurochem Res 27:601–607, doi:10.1023/A:1020215815013
Obeid LM, Linardic CM, Karolak LA, Hannun YA (1993) Programmed cell death induced by ceramide. Science 259:1769–1771, doi:10.1126/science.8456305
Hannun YA, Luberto C (2000) Ceramide in the eukaryotic stress response. Trends Cell Biol 10:73–80, doi:10.1016/S0962-8924(99)01694-3
Hla T (2003) Signaling and biological actions of sphingosine 1-phosphate. Pharmacol Res 47(5):401–407, doi:10.1016/S1043-6618(03)00046-X
Olivera A, Rosenfeldt HM, Bektas M, Wang F, Ishii I, Chun J, Milstien S, Spiegel S (2003) Sphingosine kinase type 1 induces G12/13-mediated stress fiber formation, yet promotes growth and survival independent of G protein-coupled receptors. J Biol Chem 278(47):46452–46460, doi:10.1074/jbc.M308749200
Cuvillier O, Pirianov G, Kleuser B, Vanek PG, Coso OA, Gutkind S, Spiegel S (1996) Suppression of ceramide-mediated programmed cell death by sphingosine-1-phosphate. Nature 381(6585):800–803, doi:10.1038/381800a0
Lee MJ, Thangada S, Claffey KP, Ancellin N, Liu CH, Kluk M, Volpi M, Sha’afi RI, Hla T (1999) Vascular endothelial cell adherens junction assembly and morphogenesis induced by sphingosine-1-phosphate. Cell 99(3):301–312, doi:10.1016/S0092-8674(00)81661-X
Morita Y, Perez GI, Paris F, Miranda SR, Ehleiter D, Haimovitz-Friedman A, Fuks Z, Xie Z, Reed JC, Schuchman EH, Kolesnick RN, Tilly JL (2000) Oocyte apoptosis is suppressed by disruption of the acid sphingomyelinase gene or by sphingosine-1-phosphate therapy. Nat Med 6(10):1109–1114, doi:10.1038/80442
Zelinski MB, Pau F, Murphy M, Lawson M, Fanton J, Tilly J (2006) Intraovarian delivery os sphingosine-1-phosphate protects ovarian follicles from X-irradiation damage in rhesus monkeys. Fertil Steril 86:S95, doi:10.1016/j.fertnstert.2006.07.254
Bonnaud S, Niaudet C, Pottier G, Gaugler MH, Millour J, Barbet J, Sabatier L, Paris F (2007) Sphingosine-1-phosphate protects proliferating endothelial cells from ceramide-induced apoptosis but not from DNA damage-induced mitotic death. Cancer Res 67(4):1803–1811, doi:10.1158/0008-5472.CAN-06-2802
German OL, Miranda GE, Abrahan CE, Rotstein NP (2006) Ceramide is a mediator of apoptosis in retina photoreceptors. Invest Ophthalmol Vis Sci 47:1658–1668, doi:10.1167/iovs.05-1310
Sanvicens N, Cotter TG (2006) Ceramide is the key mediator of oxidative stress-induced apoptosis in retinal photoreceptor cells. J Neurochem 98:1432–1444, doi:10.1111/j.1471-4159.2006.03977.x
Berglin L, Algvere PV, Seregard S (1997) Photoreceptor decay over time and apoptosis in experimental retinal detachment. Arch Clin Exp Ophthalmol 235:306–312, doi:10.1007/BF01739640
Mervin K, Valter K, Maslim J, Lewis G, Fisher S, Stone J (1999) Limiting photoreceptor death and deconstruction during experimental retinal detachment: the value of oxygen supplementation. Am J Ophthalmol 128:155–164, doi:10.1016/S0002-9394(99)00104-X
Francke E, Faude F, Pannicke T, Bringmann A, Eckstein P, Reichelt W, Wiedemann P, Reichenbach A (2001) Electrophysiology of rabbit Muller (glial) cells in experimental retinal detachment and PVR. Invest Ophthalmol Vis Sci 45:1072–1079
Zacks DN, Hanninen V, Pantcheva M, Ezra E, Grosskreutz C, Miller JW (2003) Caspase activation in an experimental model of retinal detachment. Invest Ophthalmol Vis Sci 44:1262–1267, doi:10.1167/iovs.02-0492
Bielawska A, Perry DK, Hannun YA (2001) Determination of ceramides and diglycerides by the diglyceride kinase assay. Anal Biochem 298:141–150, doi:10.1006/abio.2001.5342
Ames B (1966) Assay of inorganic phosphate, total phosphate and phosphatases. Methods Enzymol 8:115–118, doi:10.1016/0076-6879(66)08014-5
Kawase M, Watanabe M, Kondo T, Yabu T, Taguchi Y, Umehara H, Uchiyama T, Mizuno K, Okazaki T (2002) Increase of ceramide in adriamycin-induced HL-60 cell apoptosis: detection by a novel anti-ceramide antibody. Biochim Biophys Acta 1584(2–3):104–114
Medin JA, Takenaka T, Carpentier S (1999) Retrovirus-mediated correction of the metabolic defect in cultured Farber disease cells. Hum Gene Ther 10:1321–1329, doi:10.1089/10430349950018003
Cook B, Lewis GP, Fisher SK, Adler R (1995) Apoptotic photoreceptor degeneration in experimental retinal detachment. Invest Ophthalmol Vis Sci 36:990–996
Abler AS, Chang CJ, Ful J, Tso MO, Lam TT (1996) Photic injury triggers apoptosis of photoreceptor cells. Res Commun Mol Pathol Pharmacol 92:177–189
Shahinfar S, Edward DP, Tso MO (1991) A pathologic study of photoreceptor cell death in retinal photic injury. Curr Eye Res 10:47–59, doi:10.3109/02713689109007610
Tso MO, Zhang C, Abler AS, Chang CJ, Wong F, Chang GQ, Lam TT (1994) Apoptosis leads to photoreceptor degeneration in inherited retinal dystrophy of RCS rats. Invest Ophthalmol Vis Sci 35:2693–2699
Hafezi F, Steinbach JP, Marti A, Munz K, Wang ZQ, Wagner EF, Aguzzi A, Reme CE (1997) The absence of c-fos prevents light-induced apoptotic cell death of photoreceptors in retinal degeneration in vivo. Nat Med 3:346–349, doi:10.1038/nm0397-346
Portera-Cailliau C, Sung CH, Nathans J, Adler R (1994) Apoptotic photoreceptor cell death in mouse models of retinitis pigmentosa. Proc Natl Acad Sci USA 91:974–978, doi:10.1073/pnas.91.3.974
Anderson DH, Guerin CJ, Erickson PA, Stern WH, Fisher SK (1986) Morphological recovery in the reattached retina. Invest Ophthalmol Vis Sci 27:168–183
Guerin CJ, Lewis GP, Fisher SK, Anderson DH (1993) Recovery of photoreceptor outer segment length and analysis of membrane assembly rates in regenerating primate photoreceptor outer segments. Invest Ophthalmol Vis Sci 34:175–183
Arroyo JG, Yanf L, Bula D, Chen DF (2005) Photoreceptor apoptosis in human retinal detachment. Am J Ophthalmol 139:05–610
Zarbin MA, Green WR, Moser AB, Tiffany C (1988) Increased levels of ceramide in the retina of a patient with Farber’s disease. Arch Ophthalmol 106:1163
Watanabe M, Kitano T, Kondo T, Yabu T, Taguchi Y, Tashima M, Umehara H, Domae N, Uchiyama T, Okazaki T (2004) Increase of nuclear ceramide through caspase-3-dependent regulation of the “sphingomyelin cycle” in Fas-induced apoptosis. Cancer Res 64:1000–1007, doi:10.1158/0008-5472.CAN-03-1383
Tsugane K, Tamiya-Koizumi K, Nagino M, Nimura Y, Yoshida SA (1999) Possible role of nuclear ceramide and sphingosine in hepatocyte apoptosis in rat liver. J Hepatol 31:8–17, doi:10.1016/S0168-8278(99)80158-5
Vielhaber G, Pfeiffer S, Brade L, Lindner B, Goldmann T, Vollmer E, Hintze U, Wittern KP, Wepf R (2001) Localization of Ceramide and glucosylceramide in human epidermis by immunogold electron microscopy. J Invest Dermatol 117:1126–1136, doi:10.1046/j.0022-202x.2001.01527.x
Levade T, Jaffrezou JP (1999) Signalling sphingomyelinases: which, where, how and why? Biochim Biophys Acta 1438:1–17
Barak A, Morse LS, Goldkorn T (2001) Ceramide: a potential mediator of apoptosis in human retinal pigment epithelial cells. Invest Ophthalmol Vis Sci 42:247–254
Mathias S, Pena LA, Kolesnick RN (1998) Signal transduction of stress via ceramide. Biochem J 335:465–480
Barak A, Goldkorn T, Morse LS (2005) Laser induces apoptosis and ceramide production in human retinal pigment epithelial cells. Invest Ophthalmol Vis Sci 46:2587–2591, doi:10.1167/iovs.04-0920
Cuvillier O, Rosenthal DS, Smulson ME, Spiegel S (1998) Sphingosine 1-phosphate inhibits activation of caspases that cleave poly(ADP-ribose) polymerase and lamins during Fas- and ceramide-mediated apoptosis in Jurkat T lymphocytes. J Biol Chem 273(5):2910–2916
Edsall LC, Cuvillier O, Twitty S, Spiegel S, Milstien S (2001) Sphingosine kinase expression regulates apoptosis and caspase activation in PC12 cells. J Neurochem 76(5):1573–1584, doi:10.1046/j.1471-4159.2001.00164.x
Spiegel S, Cuvillier O, Edsall LC, Kohama T, Menzeleev R, Olah Z, Olivera A, Pirianov G, Thomas DM, Tu Z, Van Brocklyn JR, Wang F (1998) Sphingosine-1-phosphate in cell growth and cell death. Ann N Y Acad Sci 845:11–18, doi:10.1111/j.1749-6632.1998.tb09658.x
Kester M, Kolesnick R (2003) Sphingolipids as therapeutics. Pharmacol Res 47(5):365–371, doi:10.1016/S1043-6618(03)00048-3
Hancke K, Strauch O, Kissel C, Gobel H, Schafer W, Denschlag D (2007) Sphingosine 1-phosphate protects ovaries from chemotherapy-induced damage in vivo. Fertil Steril 87(1):172–177, doi:10.1016/j.fertnstert.2006.06.020
Otala M, Suomalainen L, Pentikainen MO, Kovanen P, Tenhunen M, Erkkila K, Toppari J, Dunkel L (2004) Protection from radiation-induced male germ cell loss by sphingosine-1-phosphate. Biol Reprod 70(3):759–767, doi:10.1095/biolreprod.103.021840
Nakazawa T, Hisatomi T, Nakazawa C, Noda K, Maruyama K, She H, Matsubara A, Miyahara S, Nakao S, Yin Y, Benowitz L, Hafezi-Moghadam A, Miller JW (2007) Monocyte chemoattractant protein 1 mediates retinal detachment-induced photoreceptor apoptosis. Proc Natl Acad Sci USA 104(7):2425–2430
Lewis GP, Linberg KA, Geller SF, Guérin CJ, Fisher SK (1999) Effects of the neurotrophin brain-derived neurotrophic factor in an experimental model of retinal detachment. Invest Ophthalmol Vis Sci 40(7):1530–1544
Chong DY, Boehlke CS, Zheng QD, Zhang L, Han Y, Zacks DN (2008) Interleukin-6 as a photoreceptor neuroprotectant in an experimental model of retinal detachment. Invest Ophthalmol Vis Sci 49(7):3193–3200, doi:10.1167/iovs.07-1641
Acknowledgements
The authors thank Dr. T. Okazaki (Kyoto, Japan) for providing the anti-ceramide antibody against ceramide, L. Larrié and M. Mathieu for technical assistance, and Dr. H. Etchevers for constructive comments.
Author information
Authors and Affiliations
Corresponding author
Additional information
There is no financial relationship with an organisation.
Rights and permissions
About this article
Cite this article
Ranty, ML., Carpentier, S., Cournot, M. et al. Ceramide production associated with retinal apoptosis after retinal detachment. Graefes Arch Clin Exp Ophthalmol 247, 215–224 (2009). https://doi.org/10.1007/s00417-008-0957-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00417-008-0957-6