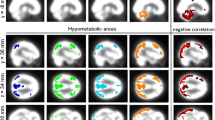
Abstract
The clinical diagnosis of Huntington’s disease (HD) is based on the motor symptoms, although these can be preceded by cognitive and behavioral changes. Biomarker studies have shown that structural imaging modalities are useful biomarkers of HD onset, while functional imaging measures have been studied less often for this purpose. Our aim was to investigate the combined value of 18-fluorodesoxyglucose (FDG)–PET and cognitive measures as biomarkers of HD onset. Twenty-two premanifest mutation carriers of HD (PMCs) and 11 healthy controls were assessed twice with FDG–PET scan, neurological and neuropsychological assessments over a 2-year interval. Seventeen PMCs had an additional third neurological evaluation, 10 years after baseline. Disease load was defined as the probability of motor onset within 5 years. Metabolism in putamen, caudate and pallidum of PMCs was significantly lower than that of controls, at both assessments. Almost half of the PMCs had converted to manifest HD 10 years later and all converters had low average or abnormal putaminal metabolism at 2 year follow-up. In contrast, all PMCs with normal putaminal metabolism at 2 year follow-up remained premanifest during the following 8 years. Furthermore, glucose metabolism of putamen explained a substantial part of the variance in disease load. A composite score of psychomotor tests contributed significantly to the prediction model as well, while cognitive performance was comparable for PMCs and controls. We conclude that in future clinical trials a combination of psychomotor tests and putaminal glucose metabolism may be used to identify PMCs close to motor onset of HD.

Similar content being viewed by others
References
The Huntington’s Disease Collaborative Research Group (1993) A novel gene containing a trinucleotide repeat that is expanded and unstable on Huntington’s disease chromosomes. Cell 72(6):971–983
Kirkwood SC, Siemers E, Hodes ME, Conneally PM, Christian JC, Foroud T (2000) Subtle changes among presymptomatic carriers of the Huntington’s disease gene. J Neurol Neurosurg Psychiatry 69(6):773
Paulsen JS, Zhao H, Stout JC, Brinkman RR, Guttman M, Ross CA, Como P, Manning C, Hayden MR, Shoulson I, Huntington Study Group (2001) Clinical markers of early disease in persons near onset of Huntington’s disease. Neurology 57(4):658–662
Biglan KM, Zhang Y, Long JD, Geschwind M, Kang GA, Killoran A, Lu W, McCusker E, Mills JA, Raymond LA, Testa C, Wojcieszek J, Paulsen JS, PREDICT-HD Investigators of the Huntington Study Group (2013) Refining the diagnosis of Huntington disease: the PREDICT-HD study. Front Aging Neurosci 5:12. doi:10.3389/fnagi.2013.00012
Andrew SE, Goldberg YP, Kremer B, Telenius H, Theilmann J, Adam S, Starr E, Squitieri F, Lin B, Kalchman MA (1993) The relationship between trinucleotide (CAG) repeat length and clinical features of Huntington’s disease. Nat Genet 4(4):398–403. doi:10.1038/ng0893-398
Duyao M, Ambrose C, Myers R, Novelletto A, Persichetti F, Frontali M, Folstein S, Ross C, Franz M, Abbott M et al (1993) Trinucleotide repeat length instability and age of onset in Huntington’s disease. Nat Genet 4(4):387–392. doi:10.1038/ng0893-387
Langbehn DR, Brinkman RR, Falush D, Paulsen JS, Hayden MR, International Huntington’s Disease Collaborative Group (2004) A new model for prediction of the age of onset and penetrance for Huntington’s disease based on CAG length. Clin Genet 65(4):267–277. doi:10.1111/j.1399-0004.2004.00241.x
Paulsen JS, Langbehn DR, Stout JC, Aylward E, Ross CA, Nance M, Guttman M, Johnson S, MacDonald M, Beglinger LJ, Duff K, Kayson E, Biglan K, Shoulson I, Oakes D, Hayden M, PREDICT-HD Investigators and Coordinators of the Huntington Study Group (2008) Detection of Huntington’s disease decades before diagnosis: the PREDICT-HD study. J Neurol Neurosurg Psychiatry 79(8):874–880
Tabrizi SJ, Langbehn DR, Leavitt BR, Roos RA, Durr A, Craufurd D, Kennard C, Hicks SL, Fox NC, Scahill RI, Borowsky B, Tobin AJ, Rosas HD, Johnson H, Reilmann R, Landwehrmeyer B, Stout JC, TRACK-HD investigators (2009) Biological and clinical manifestations of Huntington’s disease in the longitudinal TRACK-HD study: cross-sectional analysis of baseline data. Lancet Neurol 8(9):791–801. doi:10.1016/S1474-4422(09)70170-X
Stout JC, Paulsen JS, Queller S, Solomon AC, Whitlock KB, Campbell JC, Paulsen JS, Queller S, Solomon AC, Whitlock KB, Campbell JC, Carlozzi N, Duff K, Beglinger LJ, Langbehn DR, Johnson SA, Biglan KM, Aylward EH (2011) Neurocognitive signs in prodromal Huntington disease. Neuropsychology 25(1):1–14. doi:10.1037/a0020937
Tabrizi SJ, Reilmann R, Roos RA, Durr A, Leavitt B, Owen G, Jones R, Johnson H, Craufurd D, Hicks SL, Kennard C, Landwehrmeyer B, Stout JC, Borowsky B, Scahill RI, Frost C, Langbehn DR, TRACK-HD investigators (2012) Potential endpoints for clinical trials in premanifest and early Huntington’s disease in the TRACK-HD study: analysis of 24 month observational data. Lancet Neurol 11(1):42–53. doi:10.1016/S1474-4422(11)70263-0
Kuhl DE, Markham CH, Metter EJ, Riege WH, Phelps ME, Mazziotta JC (1985) Local cerebral glucose utilization in symptomatic and presymptomatic Huntington’s disease. Res Publ Assoc Res Nerv Ment Dis 63:199–209
Antonini A, Leenders KL, Spiegel R, Meier D, Vontobel P, Weigell-Weber M, Sanchez-Pernaute R, de Yébenez JG, Boesiger P, Weindl A, Maguire RP (1996) Striatal glucose metabolism and dopamine D2 receptor binding in asymptomatic gene carriers and patients with Huntington’s disease. Brain J Neurol 119(6):2085–2095
Feigin A, Leenders KL, Moeller JR, Missimer J, Kuenig G, Spetsieris P, Antonini A, Eidelberg D (2001) Metabolic network abnormalities in early Huntington’s disease: an [18F]FDG PET study. J Nucl Med 42(11):1591–1595
Van Oostrom JCH, Maguire RP, Verschuuren-Bemelmans CC, Veenma-van der Pruim J, Roos RAC, Leenders KL (2005) Striatal dopamine D2 receptors, metabolism, and volume in preclinical Huntington disease. Neurology 65(6):941–943
Feigin A, Tang C, Ma Y, Mattis P, Zgaljardic D, Guttman M, Paulsen JS, Dhawan V, Eidelberg D (2007) Thalamic metabolism and symptom onset in preclinical Huntington’s disease. Brain J Neurol 130(11):2858–2867
Paulsen JS (2009) Functional imaging in Huntington’s disease. Exp Neurol 216(2):272–277. doi:10.1016/j.expneurol.2008.12.015
Tang CC, Feigin A, Ma Y, Habeck C, Paulsen JS, Leenders KL, Teune LK, van Oostrom JCH, Guttman M, Dhawan V, Eidelberg D (2013) Metabolic network as a progression biomarker of premanifest Huntington’s disease. J Clin Investig 123(9):4076–4088. doi:10.1172/JCI69411
Van Oostrom JCH, Dekker M, Willemsen AT, De Jong BM, Roos RA, Leenders KL (2009) Changes in striatal dopamine D2 receptor binding in pre-clinical Huntington’s disease. Eur J Neurol 16(2):226–231. doi:10.1111/j.1468-1331.2008.02390.x
Huntington Study Group (1996) Unified Huntington’s disease rating scale: reliability and consistency. Mov Disord 11(2):136–142
Folstein MF, Folstein SE, McHugh PR (1975) “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res 12(3):189–198
Verhage F (1964) Intelligentie en leeftijd: Onderzoek bij Nederlanders van twaalf tot zevenenzeventig jaar (Proefschrift). Van Gorcum, Assen
Quinn N, Brown R, Craufurd D, Goldman S, Hodges J, Kieburtz K, Lindvall O, MacMillan J, Roos R (1996) Core assessment program for intracerebral transplantation in Huntington’s disease (CAPIT-HD). Mov Disord 11(2):143–150
Stroop JR (1935) Studies of interference in serial verbal reactions. J Exp Psychol 18:643–662
Reitan RM (1958) Validity of the trail making test as an indicator of organic brain damage. Percept Mot Skills 8:271–276
Smith A (1968) The symbol digit modalities test: a neuropsychologic test for economic screening of learning and other cerebral disorders. Learn Disord 3:83–91
Benton AL (1976) Multilingual aphasia examination. University of Iowa Press, Iowa City
Wechsler DA (1945) A standardized memory scale for clinical use. J Psychol 19:87–95
Holm S (1979) A simple sequentially rejective multiple test procedure. Scand J Stat 6(2):65–70
Tabrizi SJ, Scahill RI, Owen G, Durr A, Leavitt BR, Roos RA, Borowsky B, Landwehrmeyer B, Frost C, Johnson H, Craufurd D, Reilmann R, Stout JC, Langbehn DR, The TRACK-HD Investigators (2013) Predictors of phenotypic progression and disease onset in premanifest and early-stage Huntington’s disease in the TRACK-HD study: analysis of 36-month observational data. Lancet Neurol 12(7):637–649. doi:10.1016/S1474-4422(13)70088-7
Aylward EH, Brandt J, Codori AM, Mangus RS, Barta PE, Harris GJ (1994) Reduced basal ganglia volume associated with the gene for Huntington’s disease in asymptomatic at-risk persons. Neurology 44(5):823–828
Wolf RC, Vasic N, Schönfeldt-Lecuona C, Landwehrmeyer GB, Ecker D (2007) Dorsolateral prefrontal cortex dysfunction in presymptomatic Huntington’s disease: evidence from event-related fMRI. Brain nov 130(Pt 11):2845–2857 Epub 2007 sep 13
Harrington DL, Smith MM, Zhang Y, Carlozzi NE, Paulsen JS, The PREDICT-HD Investigators of the Huntington Study Group (2012) Cognitive domains that predict time to diagnosis in prodromal Huntington disease. J Neurol Neurosurg Psychiatry 83:612–619. doi:10.1136/jnnp-2011-301732
Campodonico JR, Aylward E, Codori A, Yound C, Krafft L, Magdalinski M, Ranen N, Slavney PR, Brandt J (1998) When does Huntington’s disease begin? J Int Neuropsychol Soc 4:467–473
Jurgens CK, van de Wiel L, van Es A, Grimbergen YM, Witjes-Ané MN, van der Grond J, Middelkoop HA, Roos RA (2008) Basal ganglia volume and clinical correlates in ‘preclinical’ Huntington’s disease. J Neurol 255(11):1785–1791. doi:10.1007/s00415-008-0050-4
Snowden JS, Craufurd D, Thompson J, Neary D (2002) Psychomotor, executive, and memory function in preclinical Huntington’s disease. Neuropsychol Dev Cognit Sect A 24(2):133
Stout JC, Jones R, Labuschagne I, O’Regan AM, Say MJ, Dumas EM, Queller S, Justo D, Santos RD, Coleman A, Hart EP, Dürr A, Leavitt BR, Roos RA, Langbehn DR, Tabrizi SJ, Frost C (2012) Evaluation of longitudinal 12 and 24 month cognitive outcomes in premanifest and early Huntington’s disease. J Neurol Neurosurg Psychiatry 83(7):687–694. doi:10.1136/jnnp-2011-301940
Witjes-Ané MN, Mertens B, van Vugt JP, Bachoud-Lévi AC, van Ommen G, Roos RA (2007) Longitudinal evaluation of “presymptomatic” carriers of Huntington’s disease. J Neuropsychiatry Clin Neurosci 19(3):310–317
Acknowledgments
The authors thank all participants for their effort and A.T.M. Willemsen for his help with the FDG–PET data analysis. This work was financed by the Prinses Beatrix Fonds, Project No. 99-0209.
Ethical standards
This study was approved by the local ethics committee and the research was conducted in compliance with institutional guidelines. All subjects gave their written informed consent.
Conflicts of interest
The research was conducted in compliance with institutional guidelines. There are no potential conflicts of interest in this study. This manuscript, or parts of it, has not been previously published elsewhere nor has it been submitted simultaneously for publication elsewhere.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Herben-Dekker, M., van Oostrom, J.C.H., Roos, R.A.C. et al. Striatal metabolism and psychomotor speed as predictors of motor onset in Huntington’s disease. J Neurol 261, 1387–1397 (2014). https://doi.org/10.1007/s00415-014-7350-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00415-014-7350-7