Abstract
Purpose
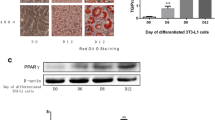
The principal function of the adipose tissue is the storage of energy in the form of triglyceride through the process of adipogenesis, as well as the provision of the stored energy through lipolysis. In the present study, we investigated the effect of hydroxytyrosol on lipolysis in 3T3-L1 adipocytes.
Methods
3T3-L1 adipocytes, used as in vitro model in this study, were treated with several concentration of hydroxytyrosol. Glycerol release was measured to identify the lipolytic rate activation. All factors activation and expression were carried out via Western blotting and qRT-PCR.
Results
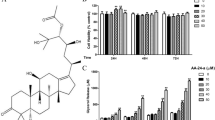
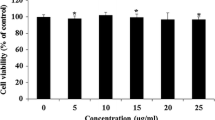
Our results showed that hydroxytyrosol, over a range of concentrations, attenuated triglyceride accumulation and stimulated glycerol release in fully differentiated adipocytes in a dose- and time-dependent manner. Moreover, hydroxytyrosol had no effect on adipocyte viability. To understand the mechanism underlying hydroxytyrosol-stimulated lipolysis, we used inhibitors of PKA, PKC, PKG, ERK1/2, and nitric oxide production. Pretreatment with a PKA inhibitor (Rp-cAMPs) and an ERK1/2 inhibitor (U0126) significantly attenuated hydroxytyrosol-stimulated lipolysis. In contrast, a PKC inhibitor (Calphostin C), 2 PKG inhibitors (KT 5823 and Rp-cGMPs), and a nitric oxide inhibitor (S-ethyl ITU) had no effect on hydroxytyrosol-stimulated lipolysis. Over the same range of concentrations, hydroxytyrosol downregulated the expression of adipose triglyceride lipase, hormone sensitive lipase (HSL), and adipogenesis-related transcription factors PPARγ and C/EBPα. In addition, hydroxytyrosol increased the phosphorylation rate of HSL at Ser563 and Ser660, as well as perilipin and ERK phosphorylation.
Conclusion
Hydroxytyrosol induced lipolysis in 3T3-L1 adipocytes via the activation of PKA and ERK1/2 pathway.






Similar content being viewed by others
References
Bartness TJ, Song CK (2007) Thematic review series: adipocyte biology. Sympathetic and sensory innervation of white adipose tissue. J Lipid Res 48:1655–1672
Lass A, Zimmermann R, Haemmerle G, Riederer M, Schoiswohl G, Schweiger M, Kienesberger P, Strauss JG, Gorkiewicz G, Zechner R (2006) Adipose triglyceride lipase-mediated lipolysis of cellular fat stores is activated by CGI-58 and defective in Chanarin-Dorfman Syndrome. Cell Metab 3:309–319
Shen WJ, Patel S, Miyoshi H, Greenberg AS, Kraemer FB (2009) Functional interaction of hormone-sensitive lipase and perilipin in lipolysis. J Lipid Res 50:2306–2313
Londos C, Brasaemle DL, Schultz CJ, Adler-Wailes DC, Levin DM, Kimmel AR, Rondinone CM (1999) On the control of lipolysis in adipocytes. Ann N Y Acad Sci 892:155–168
Holm C (2003) Molecular mechanisms regulating hormone-sensitive lipase and lipolysis. Biochem Soc Trans 31:1120–1124
Pinent M, Blade MC, Salvado MJ, Arola L, Ardevol A (2005) Intracellular mediators of procyanidin-induced lipolysis in 3T3-L1 adipocytes. J Agric Food Chem 53:262–266
Xu C, He J, Jiang H, Zu L, Zhai W, Pu S, Xu G (2009) Direct effect of glucocorticoids on lipolysis in adipocytes. Mol Endocrinol 23:1161–1170
Greenberg AS, Shen WJ, Muliro K, Patel S, Souza SC, Roth RA, Kraemer FB (2001) Stimulation of lipolysis and hormone-sensitive lipase via the extracellular signal-regulated kinase pathway. J Biol Chem 276:45456–45461
Juan CC, Chang CL, Lai YH, Ho LT (2005) Endothelin-1 induces lipolysis in 3T3-L1 adipocytes. Am J Physiol Endocrinol Metab 288:1146–1152
Lien CC, Au LC, Tsai YL, Ho LT, Juan CC (2009) Short-term regulation of tumor necrosis factor-alpha-induced lipolysis in 3T3-L1 adipocytes is mediated through the inducible nitric oxide synthase/Nitric oxide-dependent pathway. Endocrinology 150:4892–4900
Asada N, Takahashi Y, Wada M, Naito N, Uchida H, Ikeda M, Honjo M (2000) GH induced lipolysis stimulation in 3T3-L1 adipocytes stably expressing hGHR: analysis on signaling pathway and activity of 20 K hGH. Mol Cell Endocrinol 162:121–129
Fricke K, Heitland A, Maronde E (2004) Cooperative activation of lipolysis by protein kinase A and protein kinase C pathways in 3T3-L1 adipocytes. Endocrinology 145:4940–4947
Miro-Casas E, Covas MI, Fito M, Farre-Albadalejo M, Marrugat J, de la Torre R (2003) Tyrosol and hydroxytyrosol are absorbed from moderate and sustained doses of virgin olive oil in humans. Eur J Clin Nutr 57:186–190
Mukherjee S, Lekli I, Gurusamy N, Bertelli AA, Das DK (2009) Expression of the longevity proteins by both red and white wines and their cardioprotective components, resveratrol, tyrosol, and hydroxytyrosol. Free Radic Biol Med 46:573–578
Jemai H, Fki I, Bouaziz M, Bouallagui Z, El Feki A, Isoda H, Sayadi S (2008) Lipid-lowering and antioxidant effects of hydroxytyrosol and its triacetylated derivative recovered from olive tree leaves in cholesterol-fed rats. J Agric Food Chem 56:2630–2636
Petroni A, Blasevich M, Salami M, Papini N, Montedoro GF, Galli C (1995) Inhibition of platelet aggregation and eicosanoid production by phenolic components of olive oil. Thromb Res 78:151–160
Visioli F, Galli C (2002) Biological properties of olive oil phytochemicals. Crit Rev Food Sci Nutr 42:209–221
Fki I, Sahnoun Z, Sayadi S (2007) Hypocholesterolemic effects of phenolic extracts and purified hydroxytyrosol recovered from olive mill wastewater in rats fed a cholesterol-rich diet. J Agric Food Chem 55:624–631
Gonzalez-Santiago M, Martin-Bautista E, Carrero JJ, Fonolla J, Baro L, Bartolome MV, Gil-Loyzaga P, Lopez-Huertas E (2006) One-month administration of hydroxytyrosol, a phenolic antioxidant present in olive oil, to hyperlipemic rabbits improves blood lipid profile, antioxidant status and reduces atherosclerosis development. Atherosclerosis 188:35–42
Drira R, Chen S, Sakamoto K (2011) Oleuropein and hydroxytyrosol inhibit adipocyte differentiation in 3T3-L1 cells. Life Sci 89:708–716
Zou C, Shen Z (2007) One-step intracellular triglyceride extraction and quantitative measurement in vitro. J Pharmacol Toxicol Methods 56:63–66
Chomczynski P, Sacchi N (1987) Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem 162:156–159
Anthonsen MW, Ronnstrand L, Wernstedt C, Degerman E, Holm C (1998) Identification of novel phosphorylation sites in hormone-sensitive lipase that are phosphorylated in response to isoproterenol and govern activation properties in vitro. J Biol Chem 273:215–221
Shen WJ, Patel S, Natu V, Kraemer FB (1998) Mutational analysis of structural features of rat hormones-sensitive lipase. Biochemistry 37:8973–8979
Subramanian V, Rothenberg A, Gomez C, Cohen AW, Garcia A, Bhattacharyya S, Shapiro L, Dolios G, Wang R, Lisanti MP, Brasaemle DL (2004) Perilipin A mediates the reversible binding of CGI-58 to lipid droplets in 3T3-L1 adipocytes. J Biol Chem 279:42062–42071
Miyoshi H, Souza SC, Zhang HH, Strissel KJ, Christoffolete MA, Kovsan J, Rudich A, Kraemer FB, Bianco AC, Obin MS, Greenberg AS (2006) Perilipin promotes hormone-sensitive lipase-mediated adipocyte lipolysis via phosphorylation-dependent and -independent mechanisms. J Biol Chem 281:15837–15844
Miyoshi H, Perfield JW 2nd, Souza SC, Shen WJ, Zhang HH, Stancheva ZS, Kraemer FB, Obin MS, Greenberg AS (2007) Control of adipose triglyceride lipase action by serine 517 of perilipin A globally regulates protein kinase A-stimulated lipolysis in adipocytes. J Biol Chem 282:996–1002
Penfornis P, Marette A (2007) Inducible nitric oxide synthase modulates lipolysis in adipocytes. J Lipid Res 46:135–142
Soeder KJ, Snedden SK, Cao W, Della Rocca GJ, Daniel KW, Luttrell LM, Collins S (1999) The beta3-adrenergic receptor activates mitogen-activated protein kinase in adipocytes through a Gi-dependent mechanism. J Biol Chem 274:12017–12022
Greenberg AS, Shen WJ, Muliro K, Patel S, Souza SC, Roth RA, Kraemer FB (2001) Stimulation of lipolysis and hormone-sensitive lipase via the extracellular signal-regulated kinase pathway. J Biol Chem 276:45456–45461
Acknowledgments
This work was supported in part by Grants-in-Aid for Scientific Research from the Ministry of Education, Science, Sports, and Culture of Japan and a scholarship grant from the Ministry of Higher Education, Scientific Research and Technology of Tunisia. All opinions, findings, conclusions, and recommendations expressed in this publication are those of the author(s).
Conflict of interest
On behalf of all authors, the corresponding author states that there is no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Drira, R., Sakamoto, K. Hydroxytyrosol stimulates lipolysis via A-kinase and extracellular signal-regulated kinase activation in 3T3-L1 adipocytes. Eur J Nutr 53, 743–750 (2014). https://doi.org/10.1007/s00394-013-0578-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00394-013-0578-7