Abstract
Objective
This study aimed to investigate the effect exerted by teriparatide on the repair of femoral metaphyseal defect in ovariectomized rats.
Method
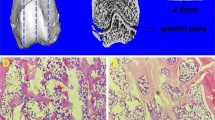
Female Sprague–Dawley rats were ovariectomized and after 3 months a critically sized defect of 3 mm in diameter—a through-hole bone defect—was drilled into each distal femur of the ovariectomized rats. The rats were injected with teriparatide (30 μg/kg) parathyroid hormone (PTH) in the peritoneum three times per week. After 4 and 8 weeks the animals were killed and the blood and bilateral femora were harvested for biochemical analysis, histopathological observation, and micro-computed tomography (CT) examination.
Results
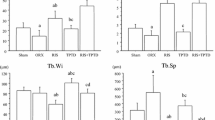
The PTH group and control group were compared 4 and 8 weeks after surgery. PTH increased bone formation in the defect area. Moreover, PTH showed the strongest effects on bone volume per total volume, trabecular number, trabecular thickness, trabecular separation, and total fluorescence-marked new bone area. Additionally, the PTH treatment group showed inhibited serum concentrations of C-terminal telopeptide of type I collagen and enhanced expression of calcium, phosphorus, and bone alkaline phosphatase.
Conclusion
Our findings suggest a positive effect of PTH on defect healing in ovariectomized rats.
Zusammenfassung
Ziel
Ziel war die Untersuchung der Wirkung von Teriparatid auf die Heilung bei einem Femurmetaphysendefekt ovarektomierter Ratten.
Methode
Bei seit 3 Monaten ovarektomierten weiblichen Sprague-Dawley(SD)-Ratten wurde ein Defekt kritischer Größe von 3 mm Durchmesser mit einer Durchgangsbohrung in jedem distalen Femur der ovarektomierten Ratten erzeugt. Den Ratten wurde 3-mal pro Woche PTH-Teriparatid (30 μg/kg) in das Peritoneum injiziert. Nach 4 bzw. 8 Wochen wurden die Tiere getötet, Blut und die Femora beidseits wurden für biochemische und histopathologische Untersuchungen sowie für eine Mikro-CT-Untersuchung entnommen.
Ergebnisse
Die Parathormon(PTH)-Gruppe und die Kontrollgruppe wurden 4 bzw. 8 Wochen nach der Operation verglichen. Durch PTH konnte die Knochenbildung im Bereich des Defekts gesteigert werden; dabei wies PTH die stärksten Wirkungen auf das Knochenvolumen pro Gesamtvolumen („bone volume per total volume“, BV/TV), die Trabekelanzahl, Trabekeldicke, Trabekelseparation sowie den gesamten fluoreszenzmarkierten Bereich der Knochenneubildung auf. Außerdem waren eine Hemmung der Expression von CTX (C-terminales Telopeptid vom Typ-I-Kollagen) sowie eine Verstärkung der Expression von Kalzium (Ca2+), Phosphat (P) und alkalischer Knochenphosphatase („bone alkaline phosphatase“, B-ALP) in der PTH-Therapie-Gruppe festzustellen.
Schlussfolgerung
Den Befunden der vorliegenden Studie zufolge ist von einem positiven Effekt von PTH auf die Defektheilung bei ovarektomierten (OVX-)Ratten auszugehen.




Similar content being viewed by others

Reference
Zhu Z, Zheng T, Lee CG, Homer RJ, Elias JA (2002) Tetracycline-controlled transcriptional regulation systems: advances and application in transgenic animal modeling. In: Seminars in cell & developmental biology, vol 2. Elsevier, pp 121–128
Riggs BL, Melton LR (1995) The worldwide problem of osteoporosis: insights afforded by epidemiology. Bone 17(5):S505–S511
Pei L, Tontonoz P (2004) Fat’s loss is bone’s gain. J Clin Invest 113(6):805
Rodan GA, Martin TJ (2000) Therapeutic approaches to bone diseases. Science 289(5484):1508–1514
Moazzaz P, Gupta MC, Gilotra MM, Gilotra MN, Maitra S, Theerajunyaporn T, Chen JL, Reddi AH, Martin RB (2005) Estrogen-dependent actions of bone morphogenetic protein—7 on spine fusion in rats. Spine 30(15):1706–1711
Yingjie H, Ge Z, Yisheng W, Ling Q, Hung W, Kwoksui L, Fuxing P (2007) Changes of microstructure and mineralized tissue in the middle and late phase of osteoporotic fracture healing in rats. Bone 41(4):631–638
Namkung-Matthai H, Appleyard R, Jansen J, Hao Lin J, Maastricht S, Swain M, Mason RS, Murrell GA, Diwan AD, Diamond T (2001) Osteoporosis influences the early period of fracture healing in a rat osteoporotic model. Bone 28(1):80–86
Reeve J, Meunier PJ, Parsons JA, Bernat M, Bijvoet OL, Courpron P, Edouard C, Klenerman L, Neer RM, Renier JC, Slovik D, Vismans FJ, Potts JT Jr (1980) Anabolic effect of human parathyroid hormone fragment on trabecular bone in involutional osteoporosis: a multicentre trial. Br Med J 280(6228):1340–1344
Mosekilde L, Gaard HC, Mcosker EJ, Wronski JT (1994) PTH has a more pronounced effect on vertebral bone mass and biomechanical competence than antiresorptive agents (estrogen and bisphosphonate) &; assessed in sexually mature, ovariectomized rats, vol 4. Elsevier, Amsterdam
Orwoll ES, Scheele WH, Paul S, Adami S, Syversen U, Diez-Perez A, Kaufman JM, Clancy AD, Gaich GA (2003) The effect of teriparatide [human parathyroid hormone (1–34)] therapy on bone density in men with osteoporosis. J Bone Miner Res 18(1):9–17. doi:10.1359/jbmr.2003.18.1.9
Neer RM, Arnaud CD, Zanchetta JR, Prince R, Gaich GA, Reginster JY, Hodsman AB, Eriksen EF, Ish-Shalom S, Genant HK, Wang O, Mitlak BH (2001) Effect of parathyroid hormone (1–34) on fractures and bone mineral density in postmenopausal women with osteoporosis. N Engl J Med 344(19):1434–1441. doi:10.1056/NEJM200105103441904
Dhillon RS, Xie C, Tyler W, Calvi LM, Awad HA, Zuscik MJ, OʼKeefe RJ, Schwarz EM (2013) PTH‐enhanced structural allograft healing is associated with decreased angiopoietin‐2–mediated arteriogenesis, mast cell accumulation, and fibrosis. J Bone Miner Res 28(3):586–597
Tao ZS, Zhou Q, Tu KK, Huang ZL, Xu H, Sun T, Lv YX, Cui W (2015) Treatment study of distal femur for parathyroid hormone (1–34) and beta-tricalcium phosphate on bone formation in critical size defects in rats. J Biomater Appl. doi:10.1177/0885328215592854
Wei L, Ke J, Prasadam I, Miron RJ, Lin S, Xiao Y, Chang J, Wu C, Zhang Y (2014) A comparative study of Sr-incorporated mesoporous bioactive glass scaffolds for regeneration of osteopenic bone defects. Osteoporos Int 25(8):2089–2096. doi:10.1007/s00198-014-2735-0
Li YF, Zhou CC, Li JH, Luo E, Zhu SS, Feng G, Hu J (2012) The effects of combined human parathyroid hormone (1–34) and zoledronic acid treatment on fracture healing in osteoporotic rats. Osteoporos Int 23(4):1463–1474. doi:10.1007/s00198-011-1751-6
Egermann M, Goldhahn J, Schneider E (2005) Animal models for fracture treatment in osteoporosis. Osteoporos Int 16(Suppl 2):S129–S138. doi:10.1007/s00198-005-1859-7
Turner AS (2001) Animal models of osteoporosis—necessity and limitations. Eur Cell Mater 1:66–81
Comelekoglu U, Bagis S, Yalin S, Ogenler O, Yildiz A, Sahin NO, Oguz I, Hatungil R (2007) Biomechanical evaluation in osteoporosis: ovariectomized rat model. Clin Rheumatol 26(3):380–384. doi:10.1007/s10067-006-0367-2
Hodsman AB, Bauer DC, Dempster DW, Dian L, Hanley DA, Harris ST, Kendler DL, McClung MR, Miller PD, Olszynski WP, Orwoll E, Yuen CK (2005) Parathyroid hormone and teriparatide for the treatment of osteoporosis: a review of the evidence and suggested guidelines for its use. Endocr Rev 26(5):688–703. doi:10.1210/er.2004-0006
Hock JM (2001) Anabolic actions of PTH in the skeletons of animals. J Musculoskelet Neuronal Interact 2(1):33–47
He YX, Zhang G, Pan XH, Liu Z, Zheng LZ, Chan CW, Lee KM, Cao YP, Li G, Wei L, Hung LK, Leung KS, Qin L (2011) Impaired bone healing pattern in mice with ovariectomy-induced osteoporosis: a drill-hole defect model. Bone 48(6):1388–1400. doi:10.1016/j.bone.2011.03.720
Cheng N, Dai J, Cheng X, Li S, Miron RJ, Wu T, Chen W, Zhang Y, Shi B (2013) Porous CaP/silk composite scaffolds to repair femur defects in an osteoporotic model. J Mater Sci Mater Med 24(8):1963–1975. doi:10.1007/s10856-013-4945-y
Uebelhart D, Bernard J, Hartmann DJ, Moro L, Roth M, Uebelhart B, Rehailia M, Mauco G, Schmitt DA, Alexandre C, Vico L (2000) Modifications of bone and connective tissue after orthostatic bedrest. Osteoporos Int 11(1):59–67. doi:10.1007/s001980050007
Lentle RG, Kruger MC (2005) Changes in mineralization and biomechanics of tibial metaphyses in splinted rats. J Appl Physiol 99(1):173–180. doi:10.1152/japplphysiol.00845.2004
Cortet B, Colin D, Dubois P, Delcambre B, Marchandise X (1995) Methods for quantitative analysis of trabecular bone structure. Rev Rhum 62(11):781–793
Boyd SK, Davison P, Muller R, Gasser JA (2006) Monitoring individual morphological changes over time in ovariectomized rats by in vivo micro-computed tomography. Bone 39(4):854–862. doi:10.1016/j.bone.2006.04.017
Hao YJ, Zhang G, Wang YS, Qin L, Hung WY, Leung K, Pei FX (2007) Changes of microstructure and mineralized tissue in the middle and late phase of osteoporotic fracture healing in rats. Bone 41(4):631–638. doi:10.1016/j.bone.2007.06.006
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Z-S. Tao, Y-X Lv, W. Cui, Z-L. Huang, K-K. Tu, Q. Zhou, T. Sun, and L. Yang state that there are no conflicts of interest.
All national guidelines on the care and use of laboratory animals have been followed and the necessary approval was obtained from the relevant authorities.
Rights and permissions
About this article
Cite this article
Tao, ZS., Lv, YX., Cui, W. et al. Effect of teriparatide on repair of femoral metaphyseal defect in ovariectomized rats. Z Gerontol Geriat 49, 423–428 (2016). https://doi.org/10.1007/s00391-015-0949-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00391-015-0949-1