Abstract
Purpose
To evaluate the efficacy of desmopressin on nocturia, quality of sleep (QoS), and health-related quality of life (HRQoL) in men with lower urinary tract symptoms suggestive of benign prostatic hyperplasia (LUTS/BPH) and nocturia due to nocturnal polyuria (NP) as the predominant symptom.
Methods
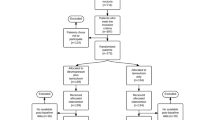
A German observational, multicenter, post-marketing surveillance study including men with LUTS/BPH and nocturia due to NP starting 3 months of desmopressin treatment.
Results
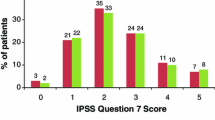
In total, 137 patients with a mean of 3.8 nocturnal voids (range 2–7) were included. Desmopressin significantly reduced the mean number of nocturnal voids by 53 %, mean IPSS nocturia question by 50 %, and the mean ratio of night/24-h urine volume by 39 % from baseline to endpoint. The hours of undisturbed sleep significantly increased by 74 %; 71 % of men reported about undisturbed sleep of ≥4 h at study end. Additionally, there was a significant reduction in the Leeds Sleep Evaluation Questionnaire score, indicating a clinically relevant QoS improvement. This was associated with an improved HRQoL, as shown by a significant improvement in both the mean IPSS-QoL question by 43 % and mean ICIQ-N nocturia problem question by 53 %. Concomitant alpha-blocker use had no effect on the efficacy of desmopressin. The incidence of adverse events was low (2.2 %). Hyponatremia was not observed in any patient. The majority of patients and physicians rated the efficacy and tolerability of desmopressin as good/very good.
Conclusions
Desmopressin is an effective and well-tolerated treatment for nocturia due to NP in patients with LUTS/BPH in daily practice under routine conditions.


Similar content being viewed by others
References
van Kerrebroeck P, Abrams P, Chaikin D, Donovan J, Fonda D, Jackson S, Jennum P, Johnson T, Lose G, Mattiasson A, Robertson G, Weiss J (2002) The standardisation of terminology in nocturia: report from the Standardisation Sub-committee of the International Continence Society. Neurourol Urodyn 21(2):179–183
Tikkinen KA, Johnson TM 2nd, Tammela TL, Sintonen H, Haukka J, Huhtala H, Auvinen A (2010) Nocturia frequency, bother, and quality of life: how often is too often? A population-based study in Finland. Eur Urol 57(3):488–496
Weiss JP, Blaivas JG, Bliwise DL, Dmochowski RR, Dubeau CE, Lowe FC, Petrou SP, Van Kerrebroeck PE, Rosen RC, Wein AJ (2011) The evaluation and treatment of nocturia: a consensus statement. BJU Int 108(1):6–21
Oelke M, Wiese B, Berges R (2014) Nocturia and its impact on health related quality of life and health care seeking behaviour in German community-dwelling men aged 50 years or older. World J Urol. doi:10.1007/s00345-014-1374-6
van Kerrebroeck P (2002) Standardization of terminology in nocturia: commentary on the ICS report. BJU Int 90(Suppl 3):16–17
Rembratt A, Norgaard JP, Andersson KE (2003) Nocturia and associated morbidity in a community-dwelling elderly population. BJU Int 92(7):726–730
Chang SC, Lin AT, Chen KK, Chang LS (2006) Multifactorial nature of male nocturia. Urology 67(3):541–544
Stanley N (2005) The physiology of sleep and the impact of ageing. Eur Urol Suppl 3:17–23
Abrams P (2005) Nocturia: the effect on sleep and related health consequences. Eur Urol Suppl 3:1–7
Asplund R (2005) Nocturia: consequences for sleep and daytime activities and associated risks. Eur Urol Suppl 3:24–32
Dijk DJ, Groeger JA, Stanley N, Deacon S (2010) Age-related reduction in daytime sleep propensity and nocturnal slow wave sleep. Sleep 33(2):211–223
Akerstedt T, Nilsson PM (2003) Sleep as restitution: an introduction. J Intern Med 254:6–12
Asplund R (1999) Mortality in the elderly in relation to nocturnal micturition. BJU Int 84(3):297–301
Bonnet MH, Arand DL (2003) Clinical effects of sleep fragmentation versus sleep deprivation. Sleep Med Rev 7:297–310
Abrams P (2005) Nocturia: the major problem in patients with lower urinary tract symptoms suggestive of benign prostatic obstruction (LUTS/BPO). Eur Urol Suppl 3:8–16
van Kerrebroeck P (2011) Nocturia: current status and future perspectives. Curr Opin Obstet Gynecol 23(5):376–385
Oelke M, Bachmann A, Descazeaud A, Emberton M, Gravas S, Michel MC, N’Dow J, Nordling J, de la Rosette JJ (2013) EAU guidelines on the treatment and follow-up of non-neurogenic male lower urinary tract symptoms including benign prostatic obstruction. Eur Urol 64(1):118–140
Eisenhardt A, Schneider T, Cruz F, Oelke M (2014) Consistent and significant improvement of nighttime voiding frequency (nocturia) with silodosin in men with LUTS suggestive of BPH: pooled analysis of three randomized, placebo-controlled, double-blind phase III studies. World J Urol. doi:10.1007/s00345-013-1228-7
Oelke M, Roehrborn CG, D’Ancona C, Wilson TH, Castro R, Manyak M (2014) Nocturia improvement in the combination of Avodart and tamsulosin (CombAT) study. World J Urol. doi:10.1007/s00345-014-1296-3
Oelke M, Roehrborn CG, D’Ancona C, Wilson TH, Castro R, Manyak M (2014) Nocturia improvement with dutasteride in men with lower urinary tract symptoms suggestive of benign prostatic hyperplasia (LUTS/BPH): a pooled analysis of three phase III studies. World J Urol. doi:10.1007/s00345-014-1316-3
Oelke M, Weiss JP, Mamoulakis C, Cox D, Ruff D, Viktrup L (2014) Effects of tadalafil on nighttime voiding (nocturia) in men with lower urinary tract symptoms suggestive of benign prostatic hyperplasia: a post hoc analysis of pooled data from four randomized, placebo-controlled clinical studies. World J Urol. doi:10.1007/s00345-014-1255-z
Oelke M, Berges R, Schlafke S, Burkart M (2014) Fixed-dose combination PRO 160/120 of sabal and urtica extracts improves nocturia in men with LUTS suggestive of BPH: re-evaluation of four controlled clinical studies. World J Urol. doi:10.1007/s00345-014-1338-x
Andersson KE, Appell R, Cardozo L (2005) Pharmalogical treatment of urinary incontinence. In: Abrams P, Cardozo L, Khoury S, Wein A (eds) Incontinence. 3rd international consultation on incontinence. Health Publication Ltd, Paris, pp 809–854
Fonda D, DuBeau C, Harari D, Ouslander JG, Palmer M, Roe B (2005) Incontinence in the frail elderly. In: Abrams P, Cardozo L, Khoury S, Wein A (eds) Incontinence (3rd international consultation on incontinence. Health Publication Ltd, Paris, pp 1163–1240
Mattiasson A, Abrams P, van Kerrebroeck P, Walter S, Weiss J (2002) Efficacy of desmopressin in the treatment of nocturia: a double-blind placebo-controlled study in men. BJU Int 89(9):855–862
van Kerrebroeck P, Rezapour M, Cortesse A, Thüroff J, Riis A, Norgaard JP (2007) Desmopressin in the treatment of nocturia: a double-blind, placebo-controlled study. Eur Urol 52(1):221–229
Lose G, Mattiasson A, Walter S, Lalos O, van Kerrebroeck P, Abrams P, Freeman R (2004) Clinical experiences with desmopressin for long-term treatment of nocturia. J Urol 172(3):1021–1025
Barry MJ, Fowler FJ Jr, O’Leary MP, Bruskewitz RC, Holtgrewe HL, Mebust WK (1995) Measuring disease-specific health status in men with benign prostatic hyperplasia. Measurement Committee of the American Urological Association. Med Care 33(4 Suppl):AS145–AS155
Rodrigues P, Meller A, Campagnari JC, Alcantara D, D’Imperio M (2004) International Prostate Symptom Score–IPSS-AUA as discriminant scale in 400 male patients with lower urinary tract symptoms (LUTS). Int Braz J Urol 30(2):135–141
McKown S, Abraham L, Coyne K, Gawlicki M, Piault E, Vats V (2010) Linguistic validation of the N-QOL (ICIQ), OAB-q (ICIQ), PPBC, OAB-S and ICIQ-MLUTSsex questionnaires in 16 languages. Int J Clin Pract 64(12):1643–1652
Parrott AC, Hindmarch I (1980) The Leeds Sleep Evaluation Questionnaire in psychopharmacological investigations—a review. Psychopharmacology 71(2):173–179
Meadows R, Venn S, Hislop J, Stanley N, Arber S (2005) Investigating couples’ sleep: an evaluation of actigraphic analysis techniques. J Sleep Res 14(4):377–386
Lose G, Lalos O, Freeman RM, van Kerrebroeck P, Nocturia Study Group (2003) Efficacy of desmopressin (Minirin) in the treatment of nocturia: a double-blind placebo-controlled study in women. Am J Obst Gynecol 189(4):1106–1113
Vande Walle J, Stockner M, Raes A, Norgaard JP (2007) Desmopressin 30 years in clinical use: a safety review. Curr Drug Saf 2:232–238
Juul KV, Klein BM, Sandstrom R, Erichsen L, Norgaard JP (2011) Gender difference in antidiuretic response to desmopressin. Am J Physiol Renal Physiol 300(5):F1116–F1122
Acknowledgments
This study was supported by an unconditional educational grant of FERRING GmbH which had no influence on the content of this publication. The authors would like to thank Dr. Jörg Schnitker, Institute for Statistics, Bielefeld, Germany for biometrical evaluation of the study data and the participating physicians for including their patients into this study and careful data collection.
Conflict of interest
Richard Berges is a lecturer, advisor and/or study participant in the field of LUTS/BPH for Astellas, GlaxoSmithKline, Ferring, Lilly, Pfizer, and Recordati. Klaus Höfner is a lecturer, advisor and/or study participant in the field of LUTS/BPH for Astellas, GlaxoSmithKline, Ferring, and Lilly. Michael Gedamke is employee of Ferring Arzneimittel GmbH in Germany. Matthias Oelke is a lecturer, advisor and/or study participant in the field of LUTS/BPH for Apogepha, Astellas, Biocompatibles, GlaxoSmithKline, Ferring, Lilly, Mundipharma, Pfizer, and Recordati.
Ethical standard
Ethics committee approval (study number FE992026_CS33) and written informed consent were obtained from all study participants. The study was designed and performed according to the German GCP recommendations and the 1964 Declaration of Helsinki.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Berges, R., Höfner, K., Gedamke, M. et al. Impact of desmopressin on nocturia due to nocturnal polyuria in men with lower urinary tract symptoms suggestive of benign prostatic hyperplasia (LUTS/BPH). World J Urol 32, 1163–1170 (2014). https://doi.org/10.1007/s00345-014-1381-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00345-014-1381-7