Abstract
Objectives
To predict residual tumor (R) classification and overall survival (OS) on preoperative MDCT in patients who underwent first-line surgery for pancreatic ductal adenocarcinoma (PDA).
Methods
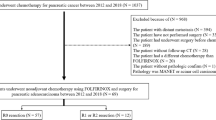
Three hundred sixteen patients with PDA who underwent MDCT and first-line surgery were included. Patients were divided into a test (n = 216) and a validation group (n = 100). The R classification was categorized into R0 (no residual tumor) and R1/R2 (microscopic/macroscopic residual tumor). We assessed the correlation between the MDCT findings and the R classification. For survival analysis, we used the Kaplan–Meier estimation and Cox proportional hazard model to determine the prognostic factors for OS. Validation of the prediction models for the R classification and OS was performed using C statistics and calibration plot.
Results
Peritumoral fat stranding (odds ratio (OR) 3.826), suspicious distant metastasis (OR 2.916), portal vein involvement (OR 2.795), and tumor size (OR 1.045) were independent predictors for residual tumor (p < .05). On survival analysis, common hepatic artery involvement (hazard ratio (HR) 5.656), R1/R2 stage (HR 2.476), and N1 stage (HR 1.745) were predictors of poor OS (p < .05). C statistics for prediction models for R classification and OS were 0.816 and 0.662, respectively. Calibration plots showed good predictive performance in a high probability of the R1/R2 stage or poor OS.
Conclusion
Preoperative MDCT is useful for predicting the R classification using the tumor size, peritumoral fat stranding, portal vein involvement, and suspicious distant metastasis, as well as for anticipating poor OS using the N1 stage, common hepatic artery involvement, and R1/R2 stage in patients with PDA.
Key Points
• Thorough assessment of the involvement of common hepatic artery or portal vein and peritumoral fat stranding is warranted for predicting prognosis in patients with pancreatic ductal adenocarcinoma.
• Not only encasement but also abutment of common hepatic artery or portal vein by tumor predicts poor prognosis after upfront surgery.
• If residual tumor or poor overall survival is anticipated on preoperative MDCT, neoadjuvant treatment can be performed.





Similar content being viewed by others
Abbreviations
- CA:
-
Celiac axis
- CHA:
-
Common hepatic artery
- MDCT:
-
Multiple detector computed tomography
- OS:
-
Overall survival
- PDA:
-
Pancreatic ductal adenocarcinoma
- PV:
-
Portal vein
- R:
-
Residual tumor
- SMA:
-
Superior mesenteric artery
- SMV:
-
Superior mesenteric vein
References
Siegel RL, Miller KD, Jemal A (2018) Cancer statistics, 2018. CA Cancer J Clin 68:7–30
Konstantinidis IT, Warshaw AL, Allen JN et al (2013) Pancreatic ductal adenocarcinoma: is there a survival difference for R1 resections versus locally advanced unresectable tumors? What is a “true” R0 resection? Ann Surg 257:731–736
Demir IE, Jager C, Schlitter AM et al (2017) R0 versus R1 resection matters after pancreaticoduodenectomy, and less after distal or total pancreatectomy for pancreatic cancer. Ann Surg. https://doi.org/10.1097/SLA.0000000000002345
Howard TJ, Krug JE, Yu J et al (2006) A margin-negative R0 resection accomplished with minimal postoperative complications is the surgeon's contribution to long-term survival in pancreatic cancer. J Gastrointest Surg 10:1338–1345 discussion 1345-1336
Sohn TA, Yeo CJ, Cameron JL et al (2000) Resected adenocarcinoma of the pancreas-616 patients: results, outcomes, and prognostic indicators. J Gastrointest Surg 4:567–579
Winter JM, Cameron JL, Campbell KA et al (2006) 1423 pancreaticoduodenectomies for pancreatic cancer: a single-institution experience. J Gastrointest Surg 10:1199–1210 discussion 1210-1191
Versteijne E, Vogel JA, Besselink MG et al (2018) Meta-analysis comparing upfront surgery with neoadjuvant treatment in patients with resectable or borderline resectable pancreatic cancer. Br J Surg. https://doi.org/10.1002/bjs.10870
Kim JH, Eun HW, Kim KW et al (2013) Diagnostic performance of MDCT for predicting important prognostic factors in pancreatic cancer. Pancreas 42:1316–1322
Karmazanovsky G, Fedorov V, Kubyshkin V, Kotchatkov A (2005) Pancreatic head cancer: accuracy of CT in determination of resectability. Abdom Imaging 30:488–500
Olivie D, Lepanto L, Billiard JS, Audet P, Lavallee JM (2007) Predicting resectability of pancreatic head cancer with multi-detector CT. Surgical and pathologic correlation. JOP 8:753–758
Lu DS, Reber HA, Krasny RM, Kadell BM, Sayre J (1997) Local staging of pancreatic cancer: criteria for unresectability of major vessels as revealed by pancreatic-phase, thin-section helical CT. AJR Am J Roentgenol 168:1439–1443
Valls C, Andia E, Sanchez A et al (2002) Dual-phase helical CT of pancreatic adenocarcinoma: assessment of resectability before surgery. AJR Am J Roentgenol 178:821–826
Tempero MA, Malafa MP, Al-Hawary M et al (2017) Pancreatic adenocarcinoma, version 2.2017, NCCN clinical practice guidelines in oncology. J Natl Compr Cancer Netw 15:1028–1061
Yamada S, Fujii T, Takami H et al (2017) Evaluation and proposal of novel resectability criteria for pancreatic cancer established by the Japan Pancreas Society. Surgery 162:784–791
Kondo N, Murakami Y, Uemura K et al (2010) Prognostic impact of perioperative serum CA 19-9 levels in patients with resectable pancreatic cancer. Ann Surg Oncol 17:2321–2329
Murakami Y, Satoi S, Sho M et al (2015) National comprehensive cancer network resectability status for pancreatic carcinoma predicts overall survival. World J Surg 39:2306–2314
Zhu L, Shi X, Xue H et al (2016) CT imaging biomarkers predict clinical outcomes after pancreatic cancer surgery. Medicine (Baltimore) 95:e2664
Matsumoto S, Mori H, Kiyonaga M et al (2012) “Peripancreatic strands appearance” in pancreatic body and tail carcinoma: evaluation by multi-detector CT with pathological correlation. Abdom Imaging 37:602–608
Al-Hawary MM, Francis IR, Chari ST et al (2014) Pancreatic ductal adenocarcinoma radiology reporting template: consensus statement of the society of abdominal radiology and the American Pancreatic Association. Gastroenterology 146:291–304 e291
Ballehaninna UK, Chamberlain RS (2012) The clinical utility of serum CA 19-9 in the diagnosis, prognosis and management of pancreatic adenocarcinoma: an evidence based appraisal. J Gastrointest Oncol 3:105–119
Imaoka H, Mizuno N, Hara K et al (2016) Prognostic impact of carcinoembryonic antigen (CEA) on patients with metastatic pancreatic cancer: a retrospective cohort study. Pancreatology 16:859–864
Gospodarowicz MK, Brierley JD, Wittekind C (2017) TNM classification of malignant tumours. John Wiley & Sons
Campbell F, Smith RA, Whelan P et al (2009) Classification of R1 resections for pancreatic cancer: the prognostic relevance of tumour involvement within 1 mm of a resection margin. Histopathology 55:277–283
Landis JR, Koch GG (1977) The measurement of observer agreement for categorical data. Biometrics 33:159–174
Harrell FE Jr, Lee KL, Mark DB (1996) Multivariable prognostic models: issues in developing models, evaluating assumptions and adequacy, and measuring and reducing errors. Stat Med 15:361–387
Kim M, Kang TW, Cha DI et al (2018) Prediction and clinical implications of portal vein/superior mesenteric vein invasion in patients with resected pancreatic head cancer: the significance of preoperative CT parameters. Clin Radiol 73:564–573
Sai M, Mori H, Kiyonaga M, Kosen K, Yamada Y, Matsumoto S (2010) Peripancreatic lymphatic invasion by pancreatic carcinoma: evaluation with multi-detector row CT. Abdom Imaging 35:154–162
Chang ST, Jeffrey RB, Patel BN et al (2016) Preoperative multidetector CT diagnosis of extrapancreatic perineural or duodenal invasion is associated with reduced postoperative survival after pancreaticoduodenectomy for pancreatic adenocarcinoma: preliminary experience and implications for patient care. Radiology 281:816–825
Kozak GM, Epstein JD, Deshmukh SP et al (2018) Common hepatic artery abutment or encasement is an adverse prognostic factor in patients with borderline and Unresectable pancreatic cancer. J Gastrointest Surg 22:288–294
Ravikumar R, Sabin C, Abu Hilal M et al (2017) Impact of portal vein infiltration and type of venous reconstruction in surgery for borderline resectable pancreatic cancer. Br J Surg 104:1539–1548
Kato H, Usui M, Isaji S et al (2013) Clinical features and treatment outcome of borderline resectable pancreatic head/body cancer: a multi-institutional survey by the Japanese Society of Pancreatic Surgery. J Hepatobiliary Pancreat Sci 20:601–610
Kim HS, Jang JY, Han Y et al (2017) Survival outcome and prognostic factors of neoadjuvant treatment followed by resection for borderline resectable pancreatic cancer. Ann Surg Treat Res 93:186–194
Gemenetzis G, Groot VP, Blair AB et al (2018) Survival in locally advanced pancreatic cancer after neoadjuvant therapy and surgical resection. Ann Surg. https://doi.org/10.1097/SLA.0000000000002753
Jeon SK, Lee JM, Joo I et al (2018) Magnetic resonance with diffusion-weighted imaging improves assessment of focal liver lesions in patients with potentially resectable pancreatic cancer on CT. Eur Radiol 28:3484–3493
Kartalis N (2018) CT and MRI of pancreatic cancer: there is no rose without a thorn! Eur Radiol 28:3482–3483
Acknowledgements
We would like to thank Bonnie Hami, MA (USA), for her editorial assistance in the preparation of this manuscript.
The authors appreciate the Medical Research Collaborating Center at Seoul National University Hospital for statistical analysis and consultation.
Funding
The authors state that this work has not received any funding.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Guarantor
The scientific guarantor of this publication is Joon Koo Han, M.D.
Conflict of interest
The authors of this manuscript declare no relationships with any companies, whose products or services may be related to the subject matter of the article.
Statistics and biometry
Medical Research Collaborating Center at Seoul National University Hospital has been consulted for statistical analysis.
Informed consent
Written informed consent was waived by the Institutional Review Board.
Ethical approval
Institutional Review Board approval was obtained (IRB No. 1609-130-796).
Methodology
• Retrospective
• Diagnostic or prognostic study
• Performed at one institution
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(DOCX 34 kb)
Rights and permissions
About this article
Cite this article
Bae, J.S., Kim, J.H., Joo, I. et al. MDCT findings predicting post-operative residual tumor and survival in patients with pancreatic cancer. Eur Radiol 29, 3714–3724 (2019). https://doi.org/10.1007/s00330-019-06140-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00330-019-06140-9