Abstract
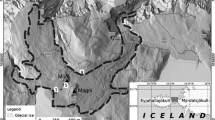
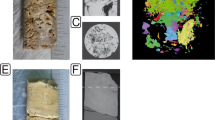
The McMurdo Dry Valleys region of eastern Antarctica is a cold desert that presents extreme challenges to life. Hypolithic microbial colonisation of the subsoil surfaces of translucent quartz rocks represent a significant source of terrestrial biomass and productivity in this region. Previous studies have described hypoliths as dominated by cyanobacteria. However, hypoliths that occur in the lower Dry Valleys such as the Miers, Garwood and Marshall Valleys are unusual as they are not necessarily cyanobacteria-dominated. These hypoliths support significant eukaryal colonisation by fungi and mosses in addition to cyanobacteria-dominated bacterial assemblages and so have considerable ecological value in this barren landscape. Here, we characterise these novel hypoliths by analysis of environmental rRNA gene sequences. The hypolithic community was demonstrated to be distinct from the surrounding soil and non-translucent rocks. Hypoliths supported cyanobacterial signatures from the Oscillatoriales and Nostocales. Other heterotrophic bacterial signatures were also recovered, and these were phylogenetically diverse and spanned 8 other bacterial phyla. Archaeal phylotypes recovered were phylogenetically affiliated with the large group of unclassified, uncultured Crenarcheota. Eukaryal phylotypes indicated that free-living ascomycetous fungi, chlorophytes and mosses (Bryum sp.) were all supported by these hypoliths, and these are thought to be responsible for the extensive eukaryotic biomass that develops around quartz rocks.






Similar content being viewed by others
References
Aislabie JM, Chhour K-L, Saul DJ, Miyauchi S, Ayton J, Paetzold RF, Balks MR (2006) Dominant bacteria in soils of Marble Point and Wright Valley, Victoria Land, Antarctica. Soil Biol Biochem 38:3041–3056
Alatalo RV (1981) Problems in the measurement of evenness in ecology. Oikos 37(2):199–204
Aller JY, Kemp PF (2008) Are archaea inherently less diverse than bacteria in the same environments? FEMS Microbiol Ecol 65:74–87
Ayton J, Aislabie J, Barker GM, Saul D, Turner S (2010) Crenarchaeota affiliated with group 1.1b are prevalent in coastal mineral soils of the Ross Sea region of Antarctica. Environ Microbiol 12:689–703
Bates ST, Berg-Lyons D, Caporaso JG, Walters WA, Knight R, Fierer N (2010) Examining the global distribution of dominant archaeal populations in soil. ISME J. doi:10.1038/ismej.2010.171
Berner T, Evenari M (1978) The influence of temperature and light penetration on the abundance of the hypolithic algae in the Negev Desert of Israel. Oecologia 33:255–260
Bradner JR, Sidhu RK, Yee B, Skotnicki ML, Selkirk PM, Nevalainen KMH (2000) A new fungal isolate, Embellisia sp., associated with the Antarctic moss Bryum argenteum. Polar Biol 23:730–732
Broady PA (1981) The ecology of sublithic terrestrial algae at the Vestfold Hills, Antarctica. Br Phycol J 16:231–240
Brown JD (1997) A rapid, non-toxic protocol for sequence-ready plasmid DNA. Tech Tips Online 2:181–183
Cary S, McDonald I, Barrett J, Cowan D (2010) On the rocks: the microbiology of Antarctic Dry Valley soils. Nature Rev 8:129–138
Cockell CS, Stokes MD (2004) Widespread colonisation by polar hypoliths. Nature 431:414
Cockell CS, Stokes MD (2006) Hypolithic colonisation of opaque rocks in the Arctic and Antarctic polar desert. Arc Antarc Alp Res 38:335–342
Cole JR, Wang Q, Cardenas E, Fish J, Chai B, Farris RJ, Kulam-Syed-Mohideen AS, McGarrell DM, Marsh T, Garrity GM, Tiedje JM (2009) The ribosomal database project: improved alignments and new tools for rRNA analysis. Nucleic Acids Res 37:D141–D145
Cowan DA, Ah Tow L (2004) Endangered Antarctic environments. Annu Rev Microbiol 58:649–690
Cowan DA, Khan N, Pointing S, Cary S (2010) Diverse hypolithic refuge communities in the McMurdo Dry Valleys. Antarc Sci 22:714–720
Cowan DA, Sohm JA, Makhalanyane TP, Capone DG, Green TGA, Cary S, Tuffin IM (2011a) Hypolithic communities: an important nitrogen source in Antarctic desert soils. Environ Microbiol Reports. doi:10.1111/j.1758-2229.2011.00266.x
Cowan DA, Pointing S, Stevens M, Cary C, Stomeo F, Tuffin M (2011b) Distribution and abiotic influences on hypolithic microbial communities in an Antarctic Dry Valley. Polar Biol 34:307–311
Felske A, Rheims H, Wolterink A, Stackebrandt E, Akkermans AD (1997) Ribosome analysis reveals prominent activity of an uncultured member of the class Actinobacteria in grassland soils. Microbiology 143:2983–2989
Friedmann EI (1982) Endolithic microorganisms in the Antarctic cold desert. Science 215:1045–1053
Friedmann EI, Ocampo R (1976) Endolithic bue-green algae in the Dry valleys: primary producers in the Antarctic desert ecosystem. Science 193:1247–1249
Friedmann EI, Hua M, Ocampo-Friedmann R (1988) Cryptoendolithic lichen and cyanobacterial communities of the Ross Desert, Antarctica. Polarforschung 58:251
Huelsenbeck JP, Bull JJ, Cunningham CW (1996) Combining data in phylogenetic analysis. TREE 11:152–158
Hughes KA, Lawley B (2003) A novel Antarctic microbial endolithic community within gypsum crusts. Environ Microbiol 5:555–565
Midwood AJ, Boutton TW (1998) Soil carbonate decomposition by acid has little effect on δ13C of organic matter. Soil Biol Biochem 30:1301–1307
Miller DN, Bryant JE, Madsen EL, Ghiorse WC (1999) Evaluation and optimisation of DNA extraction and purification procedures for soil and sediment samples. Appl Environ Microbiol 65:4715–4724
Muyzer G, Smalla K (1998) Application of denaturing gradient gel electrophoresis (DGGE) and temperature gradient gel electrophoresis (TGGE) in microbial ecology. Antonie Van Leeuwenhoek 73:127–141
Muyzer G, De Waal EC, Uitterlinden AG (1993) Profiling of complex microbial populations by denaturing gradient gel electrophoresis analysis of polymerase chain reaction-amplified genes coding for 16S rRNA. Appl Environ Microbiol 59:695–700
Niederberger TD, McDonald IR, Hacker AL, Soo RM, Barrett JE, Wall DH, Cary SC (2008) Microbial community structures of high and low productivity soils of Northern Victoria land, Antarctica. Environ Microbiol 10:1713–1724
Peet RK (1975) Relative diversity indices. Ecology 56:496–498
Piao Z, Yang L, Zhao L, Yin S (2008) Actinobacterial community structure in soils receiving long-term organic and inorganic amendments. Appl Environ Microbiol 74:526–530
Pointing SB, Warren-Rhodes KA, Lacap DC, Rhodes KL, McKay CP (2007) Hypolithic community shifts occur as a result of liquid water availability along environmental gradients in China’s hot and cold hyperarid deserts. Environ Microbiol 9:414–424
Pointing SB, Chan Y, Lacap DC, Lau MCY, Jurgens JA, Farrell RL (2009) Highly specialised microbial diversity in hyper-arid polar desert. PNAS 106:19964–19969
Rheims H, Felske A, Seufert S, Stackebrandt E (1999) Molecular monitoring of an uncultured group of the class Actinobacteria in two terrestrial environments. J Microbiol Methods 36:65–75
Schlesinger WH, Pippen JS, Wallenstein MD, Hofmockel KS, Klepeis DM, Mahall BE (2003) Community composition and photosynthesis by photoautotrophs under quartz pebbles, Southern Mojave Desert. Ecology 84:3222–3231
Smith MC, Bowman JP, Scott FJ, Line MA (2000) Sublithic bacteria associated with Antarctic quartz stones. Ant Sci 12:177–184
Smith JJ, Ah Tow L, Stafford W, Cary C, Cowan DA (2006) Bacterial diversity in three different Antarctic cold desert mineral soils. Microbial Ecol 51:413–421
Sun HS, Huppert M, Cameron RE (1978) Identification of some fungi from soil and air of Antarctica. In: Parker BC (ed) Terrestrial biology III. American Geophysical Union, USA, pp 1–26
Swofford DL (2001) PAUP*: phylogenetic analysis using parsimony (*and other methods) version 4.0b8. Sinauer, Sunderland
Taton A, Grubisic S, Brambilla E, De Wit R, Wilmotte A (2003) Cyanobacterial diversity in natural and artificial microbial mats of Lake Fryxell (McMurdo Dry Valleys, Antarctica): a morphological and molecular approach. Appl Environ Microbiol 69:5157–5169
Thomas DN (2005) Photosynthetic microbes in freezing deserts. Trends Microbiol 13:87–88
Thompson JD, Gibson TJ, Plewniak F, Jeanmougin F, Higgins DG (1997) The Clustal X Windows interface: flexible strategies for multiple sequence alignment aided by quality analysis tools. Nucleic Acids Res 24:4876–4882
Tramer EJ (1969) Bird species diversity: components of Shannon’s formula. Ecology 50:927–929
Vishniac HS (1996) Biodiversity of yeasts and filamentous microfungi in terrestrial Antarctic ecosystems. Biodivers Conserv 5:1365–1378
Warren-Rhodes KA, Rhodes KL, Pointing SB, Ewing SA, Lacap DC, Gomez-Silva B, Amundson R, Friedmann EI, McKay CP (2006) Hypolithic cyanobacteria, dry limit of photosynthesis, and microbial ecology in the hyperarid Atacama Desert. Microbial Ecol 52:389–398
Warren-Rhodes K, Rhodes KL, Pointing SB, Boyle L, Dungan J, Liu S, Zhou P, McKay CP (2007) Lithic cyanobacterial ecology across environmental gradients and spatial scales in China’s hot and cold deserts. FEMS Microbiol Ecol 61:470–482
Wong FKY, Lacap DC, Lau MCY, Aitchison JC, Cowan DA, Pointing SB (2010) Hypolithic microbial community of quartz pavement in the high-altitude tundra of central Tibet. Microb Ecol 60:730–739
Wood SA, Rueckert A, Cowan DA, Cary SC (2008) Sources of edaphic cyanobacterial diversity in the Dry Valleys of Eastern Antarctica. ISME J 2:308–320
Woodman ME (2008) Direct PCR of intact bacteria: colony PCR. In: Coico R, Kowalik T, Quarles JM, Stevenson B, Taylor RK (eds) Current protocols in microbiology. Wiley, Hoboken
Zander RH (1993) Genera of the Pottiaceae: mosses of harsh environments. Bull Buffalo Soc Nat Sci 32:1–378
Zhong L, Qiguo Z (2001) Organic carbon content and distribution in soils under different land uses in tropical and subtropical China. Plant Soil 231:175–185
Zwickl DJ (2006) Genetic algorithm approaches for the phylogenetic analysis of large biological sequence data sets under the maximum likelihood criterion. Dissertation, University of Texas
Acknowledgments
This research was undertaken under the auspices of the South African national Antarctic Programme; the New Zealand Antarctic Research Programme under Antarctica New Zealand and the Waikato University Antarctic Terrestrial Biology Research Programme. The authors gratefully acknowledge funding from the National Research Foundation (SA), the Hong Kong Research Grants Council (7733/08) and the NZ Ministry of Science and Innovation (Terrestrial Antarctic Biocomplexity Survey, nzTABS). The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Additional information
This article is an invited contribution on Global Tipping Points (Global Change and Antarctic Terrestrial Biodiversity) and part of the SCAR EBA programme. I. Hogg and D. Wall (Guest Editors).
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Khan, N., Tuffin, M., Stafford, W. et al. Hypolithic microbial communities of quartz rocks from Miers Valley, McMurdo Dry Valleys, Antarctica. Polar Biol 34, 1657–1668 (2011). https://doi.org/10.1007/s00300-011-1061-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00300-011-1061-7