Abstract
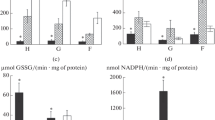
Antarctic notothenioid fishes possess high oxidative capacities, large amounts of intracellular lipid combined with biological membranes enriched in polyunsaturated fatty acids, all of which could make these animals susceptible to oxidative injury, particularly in the form of lipid peroxidation. The central objective in this study was to examine capacities for oxidative metabolism and total antioxidant defense in Antarctic and non-Antarctic notothenioids in order to test the hypothesis that the cold-bodied Antarctic fishes possess elevated activities of citrate synthase (CS), matched by a more robust antioxidant (AOX) defense, than non-Antarctic species. CS activities and total AOX capacities were measured in brain and heart of 4 Antarctic species and 2 non-Antarctic species collected on the 2004 ICEFISH cruise. While no statistical differences are found among Antarctic and non-Antarctic fishes in either CS or AOX capacities, AOX capacity in both tissues expands with CS activity among individuals measured when all species are combined. There is also a 4.5-fold greater AOX capacity, when normalized to CS activity, in brain than in heart indicating the requirement for extra AOX defense in a tissue well known for its particularly high levels of phospholipids more prone to lipid peroxidation.


Similar content being viewed by others
References
Abele D, Puntarulo S (2004) Formation of reactive oxygen species and induction of antioxidant defence systems in polar and temperate marine invertebrates and fish. Comp Biochem Physiol 138A:405–415
Cheng C-HC, Detrich HW III (2007) Molecular ecophysiology of Antarctic notothenioid fishes. Phil Trans R Soc B 362:2215–2232
Cheng C-HC, Chen L, Near T, Jin Y (2003) Functional antifreeze glycoprotein genes in temperate-water New Zealand notothenioid fish infer an Antarctic evolutionary origin. Mol Biol Evol 20:1897–1908
Coppes Petricorena ZL, Somero GN (2007) Biochemical adaptations of notothenioid fishes: comparisons between cold temperate South American and New Zealand species and Antarctic species. Comp Biochem Physiol 147A:799–807
Cosgrove JP, Church DF, Pryor WA (1987) The kinetics of the autoxidation of polyunsaturated fatty acids. Lipids 22:299–304
Crockett EL (2008) The cold but not hard fats in ectotherms: consequences of lipid restructuring on susceptibility of biological membranes to peroxidation, a review. J Comp Physiol 178B:795–809
Crockett EL, Sidell BD (1990) Some pathways of energy metabolism are cold adapted in Antarctic fishes. Physiol Zool 63:472–488
D’Autréaux B, Toledano MB (2007) ROS as signaling molecules: mechanisms that generate specificity in ROS homeostasis. Nat Rev 8:813–824
Eastman JT (1993) Antarctic fish biology. Academic Press, San Diego
Fernández DA, Calvo J, Franklin CE, Johnston IA (2000) Muscle fiber types and size distribution in sub-Antarctic notothenioid fishes. J Fish Biol 56:1295–1311
Fields PA, Somero GN (1998) Hot spots in cold adaptation: localized increases in conformational flexibility in lactate dehydrogenase A4 orthologs of Antarctic notothenioid fishes. Proc Natl Acad Sci USA 95:11476–11481
Filho DW, Giulivi C, Boveris A (1993) Antioxidant defences in marine fish—I. Teleosts. Comp Biochem Physiol 106C:409–413
Gon O, Heemstra PC (eds) (1990) Fishes of the Southern Ocean. J.L.B. Smith Institute of Ichthyology, Grahamstown
Gonzalez-Cabrera PJ, Dowd F, Pedibhotla VK, Rosario R, Stanley-Samuelson D, Petzel D (1995) Enhanced hypo-osmoregulation induced by warm-acclimation in Antarctic fish is mediated by increased gill and kidney Na+/K+-ATPase activities. J Exp Biol 198:2279–2291
Grim JM, Miles DRB, Crockett EL (2010) Temperature acclimation alters oxidative capacities and composition of membrane lipids without influencing activities of enzyme antioxidants or susceptibility to lipid peroxidation in fish muscle. J Exp Biol 213:445–552
Guderley H (2004) Metabolic responses to low temperature in fish muscle. Biol Rev Camb Philos Soc 79:409–427
Halliwell B, Gutteridge JMC (1999) Free radicals in biology and medicine. Oxford University Press, Oxford
Hofmann GE, Buckley BA, Airaksinen S, Keen JE, Somero GN (2000) Heat-shock protein expression is absent in the Antarctic fish Trematomus bernacchii (Family Nototheniidae). J Exp Biol 203:2331–2339
Hofmann GE, Lund SG, Place SP, Whitmer AC (2005) Some like it hot, some like it cold: the heat shock response is found in New Zealand but not Antarctic notothenioid fishes. J Exp Mar Biol Ecol 316:79–89
Holman RT (1954) Autoxidation of fats and related substances. In: Holman RT, Lundberg WO, Malkin T (eds) Progress in chemistry of fats and other lipids. Pergamon, London, pp 51–98
Hunt BM, Hoefling K, Cheng C-HC (2003) Annual warming episodes in seawater temperatures in McMurdo Sound in relationship to endogenous ice in notothenioid fish. Ant Sci 15:333–338
Jones CD, Anderson ME, Balushkin AV, Duhamel G, Eakin RR, Eastman JT, Kuhn KL, Lecointre G, Near TJ, North AW, Stein DL, Vacchi M, Detrich HW III (2008) Diversity, relative abundance, new locality records and population structure of Antarctic demersal fishes from the northern Scotia Arc islands and Bouvetøya. Polar Biol 31:1481–1497
Kawakatsu M, Terao J, Matsushita S (1984) Phospholipid oxidation catalyzed by ferrous ion and ascorbic acid. Agric Biol Chem 48:1275–1279
Kawall HG, Torres JJ, Sidell BD, Somero GN (2002) Metabolic cold adaptation in Antarctic fishes: evidence from enzymatic activities in brain. Mar Biol 140:279–286
Kennett JP (1982) Marine geology. Prentice-Hall, Englewood Cliffs
Leary SC, Lyons CN, Rosenberger AG, Ballantyne JS, Stillman J, Moyes CD (2003) Fiber-type differences in muscle mitochondrial profiles. Am J Physiol 285:R817–R826
Littlepage JL (1965) Oceanographic investigations in McMurdo Sound, Antarctica. Ant Res Ser 5:1–37
Logue JA, DeVries AL, Fodor E, Cossins AR (2000) Lipid compositional correlates of temperature-adaptive interspecific differences in membrane physical structure. J Exp Biol 203:2105–2115
Londraville RL, Sidell BD (1990) Ultrastructure of aerobic muscle in Antarctic fishes may contribute to maintenance of diffusive fluxes. J Exp Biol 150:205–220
McColl JD, Rossiter RJ (1952) A comparative study of the lipids of the vertebrate central nervous system. J Exp Biol 29:196–202
Near TJ (2004) Estimating divergence time of notothenioid fishes using a fossil-calibrated molecular clock. Ant Sci 16:37–44
O’Brien KM, Sidell BD (2000) The interplay among cardiac ultrastructure, metabolism and the expression of oxygen-binding proteins in Antarctic fishes. J Exp Biol 203:1287–1297
Sidell BD (1998) Intracellular oxygen diffusion: the roles of myoglobin and lipid at cold body temperature. J Exp Biol 201:1118–1127
Hansen CA, Sidell BD (1983) Atlantic hagfish cardiac muscle: metabolic basis of tolerance to anoxia. Am J Physiol 244:R356–R362
Sidell BD, Crockett EL, Driedzic WR (1995) Antarctic fish tissues preferentially catabolize monoenoic fatty acids. J Exp Zool 271:73–81
Somero GN, Childress JJ (1980) A violation of the metabolism-size scaling paradigm: activities of glycolytic enzymes in muscle increase in larger-size fish. Physiol Zool 53:322–337
Speers-Roesch B, Ballantyne JS (2005) Activities of antioxidant enzymes and cytochrome c oxidase in liver of Arctic and temperate teleosts. Comp Biochem Physiol 140A:487–494
Srere PA, Brazil A, Gonen L (1963) The citrate condensing enzyme of pigeon breast muscle and moth flight muscle. Acta Chem Scand 17:S129–S134
St-Pierre J, Buckingham JA, Roebuck SJ, Brand MD (2002) Topology of the superoxide production from different sites in the mitochondrial electron transport chain. J Biol Chem 277:44784–44790
Tocher DR, Harvie DG (1988) Fatty acid compositions of the major phosphoglycerides from fish neural tissues: (n-3) and (n-6) polyunsaturated fatty acids in rainbow trout (Salmo gairdneri) and cod (Gadhus morhua) brains and retinas. Fish Physiol Biochem 5:229–239
Viarengo A, Abele-Oeschger D, Burlando B (1998) Effects of low temperature on prooxidant processes and antioxidant defence systems in marine organisms. In: Pörtner HO, Playle RC (eds) Cold ocean physiology. Cambridge University Press, Cambridge, pp 212–235
Wang JY, Wang ZY, Kouyama T, Shibata T, Ueki T (1994) Significance of amino groups of phosphatidylethanolamine in phospholipid peroxidation of mixed liposomes. Chem Phys Lipids 71:197–203
Yang T-H, Somero GN (1993) Effects of feeding and food deprivation on oxygen consumption, muscle protein concentration and activities of energy metabolism enzymes in muscle and brain of shallow-living (Scorpaena guttata) and deep-living (Sebastolobus alascanus) scorpaenid fishes. J Exp Biol 181:213–232
Acknowledgments
I would like, first and foremost, to acknowledge and thank Dr. Bruce Sidell, who kindly collected the samples and provided an inspiration for the author over the last 25 years. My thanks to the crew and personnel of Raytheon Polar Services aboard the RVIB Nathaniel B. Palmer for their help with collections. I am also grateful for conversations with Dr. Joe Eastman, who helped fill in details about the research cruise and provided comments on an earlier version of the manuscript. Thanks also to Dr. Rebecca Holberton for use of her microplate reader. The ICEFISH cruise was supported by National Science Foundation grant OPP 01-32032 to H. William Detrich III of Northeastern University. The research was also supported by a Faculty Fellowship from Ohio University.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Crockett, E.L. Antioxidant potential is positively correlated with mitochondrial enzyme activity in Antarctic and non-Antarctic notothenioid fishes. Polar Biol 34, 113–118 (2011). https://doi.org/10.1007/s00300-010-0864-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00300-010-0864-2