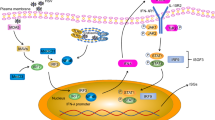
Abstract
LIGHT and herpes virus entry mediator (HVEM) comprise a ligand–receptor pair in the tumor necrosis factor superfamily. These molecules play an important role in regulating immunity, particularly in the intestinal mucosa. LIGHT also binds the lymphotoxin β receptor, and HVEM can act as a ligand for immunoglobulin family molecules, including B- and T-lymphocyte attenuator, which suppresses immune responses. Complexity in this pivotal system arises from several factors, including the non-monogamous pairing of ligands and receptors, and reverse signaling or the ability of some ligands to serve as receptors. As a result, recognition events in this fascinating network of interacting molecules can have pro- or anti-inflammatory consequences. Despite complexity, experiments we and others are carrying out are establishing rules for understanding when and in what cell types these molecules contribute to intestinal inflammation.


Similar content being viewed by others
References
Bouma G, Strober W (2003) The immunological and genetic basis of inflammatory bowel disease. Nat Rev Immunol 3(7):521–533
Sartor RB (2003) Innate immunity in the pathogenesis and therapy of IBD. J Gastroenterol 38(Suppl 15):43–47
Targan SR (2000) Biology of inflammation in Crohn’s disease: mechanisms of action of anti-TNF-a therapy. Can J Gastroenterol 14(Suppl C):13C–16C
Wang J, Fu YX (2005) Tumor necrosis factor family members and inflammatory bowel disease. Immunol Rev 204:144–155
Ware CF (2005) Network communications: lymphotoxins, LIGHT, and TNF. Annu Rev Immunol 23:787–819
Schneider K, Potter KG, Ware CF (2004) Lymphotoxin and LIGHT signaling pathways and target genes. Immunol Rev 202:49–66
Mauri D (1998) LIGHT, a new member of the TNF superfamily, and lymphotoxin α are ligands for herpesvirus entry mediator. Immunity 8:21–30
Locksley RM, Killeen N, Lenardo MJ (2001) The TNF and TNF receptor superfamilies: integrating mammalian biology. Cell 104(4):487–501
Granger SW, Butrovich KD, Houshmand P, Edwards WR, Ware CF (2001) Genomic characterization of LIGHT reveals linkage to an immune response locus on chromosome 19p13.3 and distinct isoforms generated by alternate splicing or proteolysis. J Immunol 167(9):5122–5128
Gommerman JL, Browning JL (2003) Lymphotoxin/light, lymphoid microenvironments and autoimmune disease. Nat Rev Immunol 3(8):642–655
Endres R, Alimzhanov MB, Plitz T et al (1999) Mature follicular dendritic cell networks depend on expression of lymphotoxin beta receptor by radioresistant stromal cells and of lymphotoxin beta and tumor necrosis factor by B cells. J Exp Med 189(1):159–168
Browning JL, French LE (2002) Visualization of lymphotoxin-beta and lymphotoxin-beta receptor expression in mouse embryos. J Immunol 168(10):5079–5087
Murphy M, Walter BN, Pike-Nobile L et al (1998) Expression of the lymphotoxin beta receptor on follicular stromal cells in human lymphoid tissues. Cell Death Differ 5(6):497–505
Harrop JA, Reddy M, Dede K et al (1998) Antibodies to TR2 (herpesvirus entry mediator), a new member of the TNF receptor superfamily, block T cell proliferation, expression of activation markers, and production of cytokines. J Immunol 161(4):1786–1794
Harrop JA, McDonnell PC, Brigham-Burke M et al (1998) Herpesvirus entry mediator ligand (HVEM-L), a novel ligand for HVEM/TR2, stimulates proliferation of T cells and inhibits HT29 cell growth. J Biol Chem 273(42):27548–27556
Steinberg MW, Turovskaya O, Shaikh RB et al (2008) A crucial role for HVEM and BTLA in preventing intestinal inflammation. J Exp Med 205(6):1463–1476
Schwarz BT, Wang F, Shen L et al (2007) LIGHT signals directly to intestinal epithelia to cause barrier dysfunction via cytoskeletal and endocytic mechanisms. Gastroenterology 132(7):2383–2394
Pakala SV, Ilic A, Chen L, Sarvetnick N (2001) TNF-alpha receptor 1 (p55) on islets is necessary for the expression of LIGHT on diabetogenic T cells. Clin Immunol 100(2):198–207
Morel Y, Schiano de Colella JM, Harrop J et al (2000) Reciprocal expression of the TNF family receptor herpes virus entry mediator and its ligand LIGHT on activated T cells: LIGHT down-regulates its own receptor. J Immunol 165(8):4397–4404
De Trez C, Schneider K, Potter K et al (2008) The inhibitory HVEM–BTLA pathway counter regulates lymphotoxin receptor signaling to achieve homeostasis of dendritic cells. J Immunol 180(1):238–248
Montgomery RI, Warner MS, Lum BJ, Spear PG (1996) Herpes simplex virus-1 entry into cells mediated by a novel member of the TNF/NGF receptor family. Cell 87(3):427–436
Sedy JR, Gavrieli M, Potter KG et al (2005) B and T lymphocyte attenuator regulates T cell activation through interaction with herpesvirus entry mediator. Nat Immunol 6(1):90–98
Gonzalez LC, Loyet KM, Calemine-Fenaux J et al (2005) A coreceptor interaction between the CD28 and TNF receptor family members B and T lymphocyte attenuator and herpesvirus entry mediator. Proc Natl Acad Sci USA 102(4):1116–1121
Watanabe N, Gavrieli M, Sedy JR et al (2003) BTLA is a lymphocyte inhibitory receptor with similarities to CTLA-4 and PD-1. Nat Immunol 4(7):670–679
Carter LL, Carreno BM (2003) Cytotoxic T-lymphocyte antigen-4 and programmed death-1 function as negative regulators of lymphocyte activation. Immunol Res 28(1):49–59
Han P, Goularte OD, Rufner K, Wilkinson B, Kaye J (2004) An inhibitory Ig superfamily protein expressed by lymphocytes and APCs is also an early marker of thymocyte positive selection. J Immunol 172(10):5931–5939
Hurchla MA, Sedy JR, Gavrieli M et al (2005) B and T lymphocyte attenuator exhibits structural and expression polymorphisms and is highly induced in anergic CD4+ T cells. J Immunol 174(6):3377–3385
Rooney IA, Butrovich KD, Glass AA et al (2000) The lymphotoxin-beta receptor is necessary and sufficient for LIGHT-mediated apoptosis of tumor cells. J Biol Chem 275(19):14307–14315
Cheung TC, Humphreys IR, Potter KG et al (2005) Evolutionarily divergent herpesviruses modulate T cell activation by targeting the herpesvirus entry mediator cosignaling pathway. Proc Natl Acad Sci USA 102(37):13218–13223
Compaan DM, Gonzalez LC, Tom I et al (2005) Attenuating lymphocyte activity: the crystal structure of the BTLA–HVEM complex. J Biol Chem 280(47):39553–39561
Nelson CA, Fremont MD, Sedy JR et al (2008) Structural determinants of herpesvirus entry mediator recognition by murine B and T lymphocyte attenuator. J Immunol 180(2):940–947
Ware CF (2008) The TNF superfamily—2008. Cytokine Growth Factor Rev 19(3–4):183–186
Cai G, Anumanthan A, Brown JA et al (2008) CD160 inhibits activation of human CD4+ T cells through interaction with herpesvirus entry mediator. Nat Immunol 9(2):176–185
Anumanthan A, Bensussan A, Boumsell L et al (1998) Cloning of BY55, a novel Ig superfamily member expressed on NK cells, CTL, and intestinal intraepithelial lymphocytes. J Immunol 161(6):2780–2790
Bensussan A, Gluckman E, el Marsafy S et al (1994) BY55 monoclonal antibody delineates within human cord blood and bone marrow lymphocytes distinct cell subsets mediating cytotoxic activity. Proc Natl Acad Sci USA 91(19):9136–9140
Maiza H, Leca G, Mansur IG et al (1993) A novel 80-kD cell surface structure identifies human circulating lymphocytes with natural killer activity. J Exp Med 178(3):1121–1126
Maeda M, Carpenito C, Russell RC et al (2005) Murine CD160, Ig-like receptor on NK cells and NKT cells, recognizes classical and nonclassical MHC class I and regulates NK cell activation. J Immunol 175(7):4426–4432
Zhai Y (1998) LIGHT, a novel ligand for lymphotoxin β receptor and TR2/HVEM induces apoptosis and suppresses in vivo tumor formation via gene transfer. J Clin Invest 102(6):1142–1151
Dejardin E, Droin NM, Delhase M et al (2002) The lymphotoxin-beta receptor induces different patterns of gene expression via two NF-kappaB pathways. Immunity 17(4):525
Coombes JL, Siddiqui KR, Arancibia-Carcamo CV et al (2007) A functionally specialized population of mucosal CD103+ DCs induces Foxp3+ regulatory T cells via a TGF-beta and retinoic acid-dependent mechanism. J Exp Med 204(8):1757–1764
Heo SK, Ju SA, Lee SC et al (2006) LIGHT enhances the bactericidal activity of human monocytes and neutrophils via HVEM. J Leukoc Biol 79(2):330–338
Kim WJ, Kang YJ, Koh EM et al (2005) LIGHT is involved in the pathogenesis of rheumatoid arthritis by inducing the expression of pro-inflammatory cytokines and MMP-9 in macrophages. Immunology 114(2):272–279
Hsu H, Solovyev I, Colombero A et al (1997) ATAR, a novel tumor necrosis factor receptor family member, signals through TRAF2 and TRAF5. J Biol Chem 272(21):13471–13474
Marsters SA, Ayres TM, Skubatch M et al (1997) Herpesvirus entry mediator, a member of the tumor necrosis factor receptor (TNFR) family, interacts with members of the TNFR-associated factor family and activates the transcription factors NF-kappaB and AP-1. J Biol Chem 272(22):14029–14032
Shi G, Luo H, Wan X et al (2002) Mouse T cells receive costimulatory signals from LIGHT, a TNF family member. Blood 100(9):3279–3286
Wan X, Zhang J, Luo H et al (2002) A TNF family member LIGHT transduces costimulatory signals into human T cells. J Immunol 169(12):6813–6821
Wu TH, Zhen Y, Zeng C, Yi HF, Zhao Y (2007) B and T lymphocyte attenuator interacts with CD3zeta and inhibits tyrosine phosphorylation of TCRzeta complex during T-cell activation. Immunol Cell Biol 85(8):590–595
Vendel AC, Calemine-Fenaux J, Izrael-Tomasevic A et al (2009) B and T lymphocyte attenuator regulates B cell receptor signaling by targeting Syk and BLNK. J Immunol 182(3):1509–1517
Cheung TC, Steinberg MW, Oborne LM et al (2009) Unconventional ligand activation of herpesvirus entry mediator signals cell survival. Proc Natl Acad Sci USA 106(15):6244–6249
Cohavy O, Zhou J, Granger SW, Ware CF, Targan SR (2004) LIGHT expression by mucosal T cells may regulate IFN-gamma expression in the intestine. J Immunol 173(1):251–258
Cohavy O, Zhou J, Ware CF, Targan SR (2005) LIGHT is constitutively expressed on T and NK cells in the human gut and can be induced by CD2-mediated signaling. J Immunol 174(2):646–653
Wang J, Anders RA, Wang Y et al (2005) The critical role of LIGHT in promoting intestinal inflammation and Crohn's disease. J Immunol 174(12):8173–8182
Maloy KJ, Salaun L, Cahill R et al (2003) CD4+CD25+ T(R) cells suppress innate immune pathology through cytokine-dependent mechanisms. J Exp Med 197(1):111–119
Tamada K, Shimozaki K, Chapoval AI et al (2000) LIGHT, a TNF-like molecule, costimulates T cell proliferation and is required for dendritic cell-mediated allogeneic T cell response. J Immunol 164(8):4105–4110
Tamada K, Shimozaki K, Chapoval AI et al (2000) Modulation of T-cell-mediated immunity in tumor and graft-versus-host disease models through the LIGHT co-stimulatory pathway. Nat Med 6(3):283–289
Shaikh RB, Santee S, Granger SW et al (2001) Constitutive expression of LIGHT on T cells leads to lymphocyte activation, inflammation, and tissue destruction. J Immunol 167(11):6330–6337
Wang J, Lo JC, Foster A et al (2001) The regulation of T cell homeostasis and autoimmunity by T cell-derived LIGHT. J Clin Invest 108(12):1771–1780
Probert LKJ, Corbella P, Cazlaris H, Patsavoudi E, Stephens S, Kaslaris E, Kioussis D, Kollias G (1993) Wasting, ischemia, and lymphoid abnormalities in mice expressin T cell-targeted human tumor necrosis factor transgenes. J Immunol 151(4):1894–1906
Crew MD, Effros RB, Walford RL et al (1998) Transgenic mice expressing a truncated Peromyscus leucopus TNF-alpha gene manifest an arthritis resembling ankylosing spondylitis. J Interferon Cytokine Res 18(4):219–225
Kontoyiannis D, Pasparakis M, Pizarro TT, Cominelli F, Kollias G (1999) Impaired on/off regulation of TNF biosynthesis in mice lacking TNF AU-rich elements: implications for joint and gut-associated immunopathologies. Immunity 10(3):387–398
Kontoyiannis D, Boulougouris G, Manoloukos M et al (2002) Genetic dissection of the cellular pathways and signaling mechanisms in modeled tumor necrosis factor-induced Crohn's-like inflammatory bowel disease. J Exp Med 196(12):1563–1574
Wang J, Anders RA, Wu Q et al (2004) Dysregulated LIGHT expression on T cells mediates intestinal inflammation and contributes to IgA nephropathy. J Clin Invest 113(6):826–835
Johansson-Lindbom B, Svensson M, Wurbel MA et al (2003) Selective generation of gut tropic T cells in gut-associated lymphoid tissue (GALT): requirement for GALT dendritic cells and adjuvant. J Exp Med 198(6):963–969
Berlin C, Berg EL, Briskin MJ et al (1993) Alpha 4 beta 7 integrin mediates lymphocyte binding to the mucosal vascular addressin MAdCAM-1. Cell 74(1):185–185
Spahn TW, Kucharzik T (2004) Modulating the intestinal immune system: the role of lymphotoxin and GALT organs. Gut 53(3):456–465
Banks TA, Rouse BT, Kerley MK et al (1995) Lymphotoxin-alpha-deficient mice. Effects on secondary lymphoid organ development and humoral immune responsiveness. J Immunol 155(4):1685–1693
De Togni P, Goellner J, Ruddle NH et al (1994) Abnormal development of peripheral lymphoid organs in mice deficient in lymphotoxin. Science 264(5159):703–707
Futterer A, Mink K, Luz A, Kosco-Vilbois MH, Pfeffer K (1998) The lymphotoxin beta receptor controls organogenesis and affinity maturation in peripheral lymphoid tissues. Immunity 9(1):59–70
Koni PA, Sacca R, Lawton P et al (1997) Distinct roles in lymphoid organogenesis for lymphotoxins alpha and beta revealed in lymphotoxin beta-deficient mice. Immunity 6(4):491–500
Scheu S, Alferink J, Potzel T et al (2002) Targeted disruption of LIGHT causes defects in costimulatory T cell activation and reveals cooperation with lymphotoxin beta in mesenteric lymph node genesis. J Exp Med 195(12):1613–1624
Wang J, Foster A, Chin R et al (2002) The complementation of lymphotoxin deficiency with LIGHT, a newly discovered TNF family member, for the restoration of secondary lymphoid structure and function. Eur J Immunol 32(7):1969–1979
Mackay F, Browning JL, Lawton P et al (1998) Both the lymphotoxin and tumor necrosis factor pathways are involved in experimental murine models of colitis. Gastroenterology 115(6):1464–1475
Dohi T, Rennert PD, Fujihashi K et al (2001) Elimination of colonic patches with lymphotoxin beta receptor-Ig prevents Th2 cell-type colitis. J Immunol 167(5):2781–2790
Stopfer P, Obermeier F, Dunger N et al (2004) Blocking lymphotoxin-beta receptor activation diminishes inflammation via reduced mucosal addressin cell adhesion molecule-1 (MAdCAM-1) expression and leucocyte margination in chronic DSS-induced colitis. Clin Exp Immunol 136(1):21–29
Jungbeck M, Stopfer P, Bataille F et al (2008) Blocking lymphotoxin beta receptor signalling exacerbates acute DSS-induced intestinal inflammation—opposite functions for surface lymphotoxin expressed by T and B lymphocytes. Mol Immunol 45(1):34–41
Tumanov A, Kuprash D, Lagarkova M et al (2002) Distinct role of surface lymphotoxin expressed by B cells in the organization of secondary lymphoid tissues. Immunity 17(3):239–250
Spahn TW, Herbst H, Rennert PD et al (2002) Induction of colitis in mice deficient of Peyer's patches and mesenteric lymph nodes is associated with increased disease severity and formation of colonic lymphoid patches. Am J Pathol 161(6):2273–2282
Spahn TW, Maaser C, Eckmann L et al (2004) The lymphotoxin-beta receptor is critical for control of murine Citrobacter rodentium-induced colitis. Gastroenterology 127(5):1463–1473
Clayburgh DR, Musch MW, Leitges M, Fu YX, Turner JR (2006) Coordinated epithelial NHE3 inhibition and barrier dysfunction are required for TNF-mediated diarrhea in vivo. J Clin Invest 116(10):2682–2694
Kang HS, Chin RK, Wang Y et al (2002) Signaling via LTbetaR on the lamina propria stromal cells of the gut is required for IgA production. Nat Immunol 3(6):576–582
Yu P, Lee Y, Liu W et al (2004) Priming of naive T cells inside tumors leads to eradication of established tumors. Nat Immunol 5(2):141–149
Granger SW, Rickert S (2003) LIGHT–HVEM signaling and the regulation of T cell-mediated immunity. Cytokine Growth Factor Rev 14(3–4):289–296
Xu Y, Flies AS, Flies DB et al (2007) Selective targeting of the LIGHT–HVEM co-stimulatory system for the treatment of graft-versus-host disease. Blood 109:4097–4101
Morel Y, Truneh A, Costello RT, Olive D (2003) LIGHT, a new TNF superfamily member, is essential for memory T helper cell-mediated activation of dendritic cells. Eur J Immunol 33(11):3213–3219
Morel Y, Truneh A, Sweet RW, Olive D, Costello RT (2001) The TNF superfamily members LIGHT and CD154 (CD40 ligand) costimulate induction of dendritic cell maturation and elicit specific CTL activity. J Immunol 167(5):2479–2486
Fan Z, Yu P, Wang Y et al (2006) NK-cell activation by LIGHT triggers tumor-specific CD8+ T-cell immunity to reject established tumors. Blood 107(4):1342–1351
Wang Y, Subudhi SK, Anders RA et al (2005) The role of herpesvirus entry mediator as a negative regulator of T cell-mediated responses. J Clin Invest 115(3):711–717
Tao R, Wang L, Han R et al (2005) Differential effects of B and T lymphocyte attenuator and programmed death-1 on acceptance of partially versus fully MHC-mismatched cardiac allografts. J Immunol 175(9):5774–5782
Deppong C, Juehne TI, Hurchla M et al (2006) Cutting edge: B and T lymphocyte attenuator and programmed death receptor-1 inhibitory receptors are required for termination of acute allergic airway inflammation. J Immunol 176(7):3909–3913
Krieg C, Boyman O, Fu YX, Kaye J (2007) B and T lymphocyte attenuator regulates CD8(+) T cell-intrinsic homeostasis and memory cell generation. Nat Immunol 8:162–171
Tao R, Wang L, Murphy KM, Fraser CC, Hancock WW (2008) Regulatory T cell expression of herpesvirus entry mediator suppresses the function of B and T lymphocyte attenuator-positive effector T cells. J Immunol 180(10):6649–6655
Hurchla MA, Sedy JR, Murphy KM (2007) Unexpected role of B and T lymphocyte attenuator in sustaining cell survival during chronic allostimulation. J Immunol 178(10):6073–6082
Deppong C, Degnan JM, Murphy TL, Murphy KM, Green JM (2008) B and T lymphocyte attenuator regulates T cell survival in the lung. J Immunol 181(5):2973–2979
Shao L, Serrano D, Mayer L (2001) The role of epithelial cells in immune regulation in the gut. Semin Immunol 13(3):163–176
Laouar A, Haridas V, Vargas D et al (2005) CD70+ antigen-presenting cells control the proliferation and differentiation of T cells in the intestinal mucosa. Nat Immunol 6(7):698–706
Uhlig HH, McKenzie BS, Hue S et al (2006) Differential activity of IL-12 and IL-23 in mucosal and systemic innate immune pathology. Immunity 25(2):309–318
Corazza N, Eichenberger S, Eugster HP, Mueller C (1999) Nonlymphocyte-derived tumor necrosis factor is required for induction of colitis in recombination activating gene (RAG) 2(−/−) mice upon transfer of CD4(+) CD45RB(hi) T cells. J Exp Med 190(10):1479–1492
Mucida D, Park Y, Kim G et al (2007) Reciprocal TH17 and regulatory T cell differentiation mediated by retinoic acid. Science 317(5835):256–260
Sun CM, Hall JA, Blank RB et al (2007) Small intestine lamina propria dendritic cells promote de novo generation of Foxp3 T reg cells via retinoic acid. J Exp Med 204(8):1775–1785
Mucida D, Park Y, Cheroutre H (2009) From the diet to the nucleus: vitamin A and TGF-beta join efforts at the mucosal interface of the intestine. Semin Immunol 21(1):14–21
Denning TL, Wang YC, Patel SR, Williams IR, Pulendran B (2007) Lamina propria macrophages and dendritic cells differentially induce regulatory and interleukin 17-producing T cell responses. Nat Immunol 8(10):1086–1094
Acknowledgments
This work was supported by grants from the National Institutes of Health AI61516 to M.K. and AI06789001 to C.F.W.; by a NIDDK postdoctoral fellowship (DK082249) to J-W. S; by a Research Fellowship Award from the Crohn’s & Colitis Foundation of America to M.W.S. and by the University of California, San Diego, Digestive Diseases Research Development Center (DK 080506). The authors have no conflicting financial interests. This is manuscript number 1143 from the La Jolla Institute for Allergy and Immunology.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Steinberg, M.W., Shui, JW., Ware, C.F. et al. Regulating the mucosal immune system: the contrasting roles of LIGHT, HVEM, and their various partners. Semin Immunopathol 31, 207–221 (2009). https://doi.org/10.1007/s00281-009-0157-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00281-009-0157-4