Abstract
Purpose
To evaluate the feasibility and efficacy of neoadjuvant chemotherapy involving docetaxel and cisplatin followed by intensity-modulated radiotherapy (IMRT) with concurrent cisplatin in patients with newly diagnosed stage III to IVB nasopharyngeal carcinoma (NPC).
Methods
Docetaxel (75 mg/m2 on day 1) and cisplatin (75 mg/m2 on day 1) were administered on a 3-week cycle for 2 courses, followed by radical IMRT (72 Gy/33F/6.5–7 W) with concurrent cisplatin (75 mg/m2, on day 1) every 3 weeks for 2 cycles.
Results
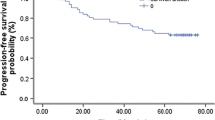
From June 2008 to October 2010, forty-six patients were recruited in this trial. Forty-five patients completed neoadjuvant setting, and all patients completed planned concurrent chemoradiotherapy (CCRT). The complete and partial response rates were 28.3 and 56.5 % after neoadjuvant chemotherapy, and 91.3, 8.7 % after CCRT, respectively. After median follow-up of 26 months (range 12–39 months), one patient experienced local recurrence and 4 patients developed distant metastasis. The 3-year overall survival and progression-free survival rate were 94.1 and 72.7 %, respectively. Neutropenia (37.0 %) and vomiting (28.3 %) were the most common Grade 3–4 adverse effects during neoadjuvant course, while mucositis (30.4 %), xerostomia (30.4 %) and radiodermatitis (21.7 %) were the most common Grades 3 acute toxicities during CCRT. Xerostomia (73.9 %), dysphagia (56.5 %), hear loss (30.4 %) and skin reaction (21.7 %) were the common Grade 1–2 late effects. There were no Grades 3–4 late toxicities.
Conclusions
The protocol of neoadjuvant docetaxel and cisplatin followed by IMRT with concurrent cisplatin was well tolerated, with outstanding compliance and efficacy in locally advanced NPC, which deserved further follow-up.


Similar content being viewed by others
Reference
Geara FB, Sanguineti G, Tucker SL et al (1997) Carcinoma of the nasopharynx treated by radiotherapy alone: determinants of distant metastasis and survival. Radiother Oncol 43:53–61
Chan AT, Teo PM, Leung TW et al (1995) A prospective randomized study of chemotherapy adjunctive to definitive radiotherapy in advanced nasopharyngeal carcinoma. Int J Radiat Oncol Biol Phys 33:569–577
Hareyama M, Sakata K, Shirato H et al (2002) A prospective, randomized trial comparing neoadjuvant chemotherapy with radiotherapy alone in patients with advanced nasopharyngeal carcinoma. Cancer 94:2217–2223
Ma J, Mai HQ, Hong MH et al (2001) Results of a prospective randomized trial comparing neoadjuvant chemotherapy plus radiotherapy with radiotherapy alone in patients with locoregionally advanced nasopharyngeal carcinoma. J Clin Oncol 19:1350–1357
Chua DT, Sham JS, Choy D et al (1998) Preliminary report of the Asian-Oceanian Clinical Oncology Association randomized trial comparing cisplatin and epirubicin followed by radiotherapy versus radiotherapy alone in the treatment of patients with locoregionally advanced nasopharyngeal carcinoma. Asian-Oceanian Clinical Oncology Association Nasopharynx Cancer Study Group. Cancer 83:2270–2283
Institute Gustave Roussy RCDV (1996) Preliminary results of a randomized trial comparing neoadjuvant chemotherapy (cisplatin, epirubicin, bleomycin) plus radiotherapy vs. radiotherapy alone in stage IV(> or = N2, M0) undifferentiated nasopharyngeal carcinoma: a positive effect on progression-free survival. International Nasopharynx Cancer Study Group. VUMCA I trial. Int J Radiat Oncol Biol Phys 35:463–469
Al-Sarraf M, LeBlanc M, Giri PG et al (1998) Chemoradiotherapy versus radiotherapy in patients with advanced nasopharyngeal cancer: phase III randomized intergroup study 0099. J Clin Oncol 16:1310–1317
Lee AW, Lau WH, Tung SY et al (2005) Preliminary results of a randomized study on therapeutic gain by concurrent chemotherapy for regionally-advanced nasopharyngeal carcinoma: NPC-9901 Trial by the Hong Kong Nasopharyngeal Cancer Study Group. J Clin Oncol 23:6966–6975
Lin JC, Jan JS, Hsu CY et al (2003) Phase III study of concurrent chemoradiotherapy versus radiotherapy alone for advanced nasopharyngeal carcinoma: positive effect on overall and progression-free survival. J Clin Oncol 21:631–637
Langendijk JA, Leemans CR, Buter J et al (2004) The additional value of chemotherapy to radiotherapy in locally advanced nasopharyngeal carcinoma: a meta-analysis of the published literature. J Clin Oncol 22:4604–4612
Baujat B, Audry H, Bourhis J et al (2006) Chemotherapy in locally advanced nasopharyngeal carcinoma: an individual patient data meta-analysis of eight randomized trials and 1753 patients. Int J Radiat Oncol Biol Phys 64:47–56
Zhang L, Zhao C, Ghimire B et al (2010) The role of concurrent chemoradiotherapy in the treatment of locoregionally advanced nasopharyngeal carcinoma among endemic population: a meta-analysis of the phase III randomized trials. BMC Cancer 10:558
Chan AT, Teo PM, Ngan RK et al (2002) Concurrent chemotherapy-radiotherapy compared with radiotherapy alone in locoregionally advanced nasopharyngeal carcinoma: progression-free survival analysis of a phase III randomized trial. J Clin Oncol 20:2038–2044
Rischin D, Corry J, Smith J et al (2002) Excellent disease control and survival in patients with advanced nasopharyngeal cancer treated with chemoradiation. J Clin Oncol 20:1845–1852
Chan AT, Ma BB, Lo YM et al (2004) Phase II study of neoadjuvant carboplatin and paclitaxel followed by radiotherapy and concurrent cisplatin in patients with locoregionally advanced nasopharyngeal carcinoma: therapeutic monitoring with plasma Epstein-Barr virus DNA. J Clin Oncol 22:3053–3060
Xu T, Hu C, Zhu G et al (2012) Preliminary results of a phase III randomized study comparing chemotherapy neoadjuvantly or concurrently with radiotherapy for locoregionally advanced nasopharyngeal carcinoma. Med Oncol 29:272–278
Sun Y, Tang LL, Chen L et al (2012) Promising treatment outcomes of intensity-modulated radiation therapy for nasopharyngeal carcinoma patients with N0 disease according to the seventh edition of the AJCC staging system. BMC Cancer 12:68
Tsang RK, Kwong DL, Ho AC et al (2012) Long-term hearing results and otological complications of nasopharyngeal carcinoma patients: comparison between treatment with conventional two-dimensional radiotherapy and intensity-modulated radiotherapy. ORL J Otorhinolaryngol Relat Spec 74:228–233
Hui EP, Ma BB, Leung SF et al (2009) Randomized phase II trial of concurrent cisplatin-radiotherapy with or without neoadjuvant docetaxel and cisplatin in advanced nasopharyngeal carcinoma. J Clin Oncol 27:242–249
Therasse P, Arbuck SG, Eisenhauer EA et al (2000) New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst 92:205–216
Trotti A, Colevas AD, Setser A et al (2003) CTCAE v3.0: development of a comprehensive grading system for the adverse effects of cancer treatment. Semin Radiat Oncol 13:176–181
Cox JD, Stetz J, Pajak TF (1995) Toxicity criteria of the Radiation Therapy Oncology Group (RTOG) and the European Organization for Research and Treatment of Cancer (EORTC). Int J Radiat Oncol Biol Phys 31:1341–1346
Lee NY, Le QT (2008) New developments in radiation therapy for head and neck cancer: intensity-modulated radiation therapy and hypoxia targeting. Semin Oncol 35:236–250
Johnson FM, Garden A, Palmer JL et al (2004) A phase II study of docetaxel and carboplatin as neoadjuvant therapy for nasopharyngeal carcinoma with early T status and advanced N status. Cancer 100:991–998
Genet D, Cupissol D, Calais G et al (2004) Docetaxel plus 5-fluorouracil in locally recurrent and/or metastatic squamous cell carcinoma of the head and neck: a phase II multicenter study. Am J Clin Oncol 27:472–476
Ngeow J, Lim WT, Leong SS et al (2011) Docetaxel is effective in heavily pretreated patients with disseminated nasopharyngeal carcinoma. Ann Oncol 22:718–722
Lu X, Guo X, Hong MH et al (2010) Comparison of the short-term efficacy of two inductive chemotherapy regimens for locally advanced nasopharyngeal caricinoma: docetaxal plus carboplatin versus 5-fluorouracil plus carboplatin. Chin J Cancer 29:140–144
Ekenel M, Keskin S, Basaran M et al (2011) Induction chemotherapy with docetaxel and cisplatin is highly effective for locally advanced nasopharyngeal carcinoma. Oral Oncol 47:660–664
Goto Y, Kodaira T, Fuwa N et al (2013) Alternating chemoradiotherapy in patients with nasopharyngeal cancer: prognostic factors and proposal for individualization of therapy. J Radiat Res. 54:98–107
Wee J, Tan EH, Tai BC et al (2005) Randomized trial of radiotherapy versus concurrent chemoradiotherapy followed by adjuvant chemotherapy in patients with American Joint Committee on Cancer/International Union against cancer stage III and IV nasopharyngeal cancer of the endemic variety. J Clin Oncol 23:6730–6738
Airoldi M, Gabriele P, Gabriele AM et al (2011) Induction chemotherapy with carboplatin and taxol followed by radiotherapy and concurrent weekly carboplatin + taxol in locally advanced nasopharyngeal carcinoma. Cancer Chemother Pharmacol 67:1027–1034
Chen A, Lee N, Yang C et al (2010) Comparison of intensity-modulated radiotherapy using helical tomotherapy and segmental multileaf collimator-based techniques for nasopharyngeal carcinoma: dosimetric analysis incorporating quality assurance guidelines from RTOG 0225. Technol Cancer Res Treat 9:291–298
Lee N, Harris J, Garden AS et al (2009) Intensity-modulated radiation therapy with or without chemotherapy for nasopharyngeal carcinoma: radiation therapy oncology group phase II trial 0225. J Clin Oncol 27:3684–3690
Xiao WW, Huang SM, Han F et al (2011) Local control, survival, and late toxicities of locally advanced nasopharyngeal carcinoma treated by simultaneous modulated accelerated radiotherapy combined with cisplatin concurrent chemotherapy: long-term results of a phase 2 study. Cancer 117:1874–1883
Lai SZ, Li WF, Chen L et al (2011) How does intensity-modulated radiotherapy versus conventional two-dimensional radiotherapy influence the treatment results in nasopharyngeal carcinoma patients? Int J Radiat Oncol Biol Phys 80:661–668
Kwong DL, Sham JS, Leung LH et al (2006) Preliminary results of radiation dose escalation for locally advanced nasopharyngeal carcinoma. Int J Radiat Oncol Biol Phys 64:374–381
Peng G, Wang T, Yang KY et al (2012) A prospective, randomized study comparing outcomes and toxicities of intensity-modulated radiotherapy vs. conventional two-dimensional radiotherapy for the treatment of nasopharyngeal carcinoma. Radiother Oncol 104:286–293
Acknowledgments
We thank M.D. Jia De Lu for critical review of the manuscript. This study was supported by the Science and Technology Planning Project of Hubei Province, China (2009BCC005).
Conflict of interest
All the authors disclosed no conflicts of interest.
Author information
Authors and Affiliations
Corresponding author
Additional information
Ya Hua Zhong and Jing Dai contributed equally to this work.
Rights and permissions
About this article
Cite this article
Zhong, Y.H., Dai, J., Wang, X.Y. et al. Phase II trial of neoadjuvant docetaxel and cisplatin followed by intensity-modulated radiotherapy with concurrent cisplatin in locally advanced nasopharyngeal carcinoma. Cancer Chemother Pharmacol 71, 1577–1583 (2013). https://doi.org/10.1007/s00280-013-2157-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00280-013-2157-2