Abstract
Purpose
Cisplatin is one of the most potent chemotherapeutic agents used to treat cancer. However, cisplatin-induced nephrotoxicity, which is partly caused by oxidative damage, is a serious problem. We previously showed that murine embryonic fibroblasts deficient in Peroxiredoxin I (Prx I), a major Nrf2-linked anti-oxidant enzyme, are susceptible to cisplatin-induced cytotoxicity. In the present study, we examined the role of Prx I against cisplatin-induced renal injury in vivo using Prx I-null mice.
Methods
Prx I-null mice and wild-type (WT) mice were given an intraperitoneal injection of cisplatin, and tissues were removed and evaluated histopathologically. In addition, gene and protein expression of efflux transporters was analyzed.
Results
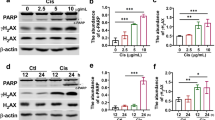
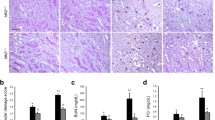
In contrast to an in vitro cell study, Prx I-null mice exhibited less cisplatin-induced renal damage than WT mice in histological and blood biochemical analyses. Moreover, Prx I-null mice showed a higher clearance rate of cisplatin than WT mice following intraperitoneal cisplatin injection. Consistent with these results, Prx I-null mice exhibited higher expression of renal efflux transporters Mrp2 and Mrp4 compared with WT mice under both basal and the cisplatin-induced conditions. We suggest the enhanced transcriptional activity of c-Myc in Prx I-null mice may partly contribute the enhanced expression of renal efflux transporters.
Conclusion
In summary, the enhanced clearance rate of cisplatin significantly attenuates nephrotoxicity in Prx I-null mice.






Similar content being viewed by others
Abbreviations
- ARF:
-
Acute renal failure
- BUN:
-
Blood urea nitrogen
- GSH:
-
Glutathione
- MDR:
-
Multidrug resistance
- Mrp:
-
Multidrug resistance-associated protein
- Nrf2:
-
Nuclear factor-E2-related factor-2
- pAb:
-
Polyclonal antibody
- Prx:
-
Peroxiredoxin
- ROS:
-
Reactive oxygen species
- TRX:
-
Thioredoxin
References
Aleksunes LM, Augustine LM, Scheffer GL, Cherrington NJ, Manautou JE (2008) Renal xenobiotic transporters are differentially expressed in mice following cisplatin treatment. Toxicology 250:82–88
Aleksunes LM, Goedken MJ, Rockwell CE, Thomale J, Manautou JE, Klaassen CD (2010) Transcriptional regulation of renal cytoprotective genes by Nrf2 and its potential use as a therapeutic target to mitigate cisplatin-induced nephrotoxicity. J Pharmacol Exp Ther 335:2–12
Borst P, Evers R, Kool M, Wijnholds J (2000) A family of drug transporters: the multidrug resistance-associated proteins. J Natl Cancer Inst 92:1295–1302
Boulikas T, Vougiouka M (2004) Recent clinical trials using cisplatin, carboplatin and their combination chemotherapy drugs (review). Oncol Rep 11:559–595
Boyer JL (2005) Nuclear receptor ligands: rational and effective therapy for chronic cholestatic liver disease? Gastroenterology 129:735–740
Butterfield LH, Merino A, Golub SH, Shau H (1999) From cytoprotection to tumor suppression: the multifactorial role of peroxiredoxins. Antioxid Redox Signal 1:385–402
Chae HZ, Kim HJ, Kang SW, Rhee SG (1999) Characterization of three isoforms of mammalian peroxiredoxin that reduce peroxides in the presence of thioredoxin. Diabetes Res Clin Pract 45:101–112
Chang TS, Jeong W, Choi SY, Yu S, Kang SW, Rhee SG (2002) Regulation of peroxiredoxin I activity by Cdc2-mediated phosphorylation. J Biol Chem 277:25370–25376
Cole SP, Bhardwaj G, Gerlach JH, Mackie JE, Grant CE, Almquist KC, Stewart AJ, Kurz EU, Duncan AM, Deeley RG (1992) Overexpression of a transporter gene in a multidrug-resistant human lung cancer cell line. Science 258:1650–1654
Daugaard G, Abildgaard U (1989) Cisplatin nephrotoxicity. A review. Cancer Chemother Pharmacol 25:1–9
Demeule M, Brossard M, Beliveau R (1999) Cisplatin induces renal expression of P-glycoprotein and canalicular multispecific organic anion transporter. Am J Physiol 277:F832–F840
Egler RA, Fernandes E, Rothermund K, Sereika S, de Souza-Pinto N, Jaruga P, Dizdaroglu M, Prochownik EV (2005) Regulation of reactive oxygen species, DNA damage, and c-Myc function by peroxiredoxin 1. Oncogene 24:8038–8050
Hofmann B, Hecht HJ, Flohe L (2002) Peroxiredoxins. Biol Chem 383:347–364
Huang Q, Dunn RT 2nd, Jayadev S, DiSorbo O, Pack FD, Farr SB, Stoll RE, Blanchard KT (2001) Assessment of cisplatin-induced nephrotoxicity by microarray technology. Toxicol Sci 63:196–207
Ishii T, Itoh K, Takahashi S, Sato H, Yanagawa T, Katoh Y, Bannai S, Yamamoto M (2000) Transcription factor Nrf2 coordinately regulates a group of oxidative stress-inducible genes in macrophages. J Biol Chem 275:16023–16029
Ishii T, Yamada M, Sato H, Matsue M, Taketani S, Nakayama K, Sugita Y, Bannai S (1993) Cloning and characterization of a 23-kDa stress-induced mouse peritoneal macrophage protein. J Biol Chem 268:18633–18636
Kang KW, Im YB, Go WJ, Han HK (2009) c-Myc amplification altered the gene expression of ABC- and SLC-transporters in human breast epithelial cells. Mol Pharm 6:627–633
Ma D, Warabi E, Yanagawa T, Kimura S, Harada H, Yamagata K, Ishii T (2009) Peroxiredoxin I plays a protective role against cisplatin cytotoxicity through mitogen activated kinase signals. Oral Oncol 45:1037–1043
Okada K, Shoda J, Kano M, Suzuki S, Ohtake N, Yamamoto M, Takahashi H, Utsunomiya H, Oda K, Sato K, Watanabe A, Ishii T, Itoh K, Yokoi T, Yoshizato K, Sugiyama Y, Suzuki H (2007) Inchinkoto, a herbal medicine, and its ingredients dually exert Mrp2/MRP2-mediated choleresis and Nrf2-mediated antioxidative action in rat livers. Am J Physiol Gastrointest Liver Physiol 292:G1450–G1463
Okada K, Shoda J, Taguchi K, Maher JM, Ishizaki K, Inoue Y, Ohtsuki M, Goto N, Takeda K, Utsunomiya H, Oda K, Warabi E, Ishii T, Osaka K, Hyodo I, Yamamoto M (2008) Ursodeoxycholic acid stimulates Nrf2-mediated hepatocellular transport, detoxification, and antioxidative stress systems in mice. Am J Physiol Gastrointest Liver Physiol 295:G735–G747
Pabla N, Dong Z (2008) Cisplatin nephrotoxicity: mechanisms and renoprotective strategies. Kidney Int 73:994–1007
Pyndiah S, Tanida S, Ahmed KM, Cassimere EK, Choe C, Sakamuro D (2011) c-MYC suppresses BIN1 to release poly(ADP-ribose) polymerase 1: a mechanism by which cancer cells acquire cisplatin resistance. Sci Signal 4:ra19
Royer B, Guardiola E, Polycarpe E, Hoizey G, Delroeux D, Combe M, Chaigneau L, Samain E, Chauffert B, Heyd B, Kantelip JP, Pivot X (2005) Serum and intraperitoneal pharmacokinetics of cisplatin within intraoperative intraperitoneal chemotherapy: influence of protein binding. Anticancer Drugs 16:1009–1016
Shord SS, Thompson DM, Krempl GA, Hanigan MH (2006) Effect of concurrent medications on cisplatin-induced nephrotoxicity in patients with head and neck cancer. Anticancer Drugs 17:207–215
Uwayama J, Hirayama A, Yanagawa T, Warabi E, Sugimoto R, Itoh K, Yamamoto M, Yoshida H, Koyama A, Ishii T (2006) Tissue Prx I in the protection against Fe-NTA and the reduction of nitroxyl radicals. Biochem Biophys Res Commun 339:226–231
Walker TL, White JD, Esdale WJ, Burton MA, DeCruz EE (1996) Tumour cells surviving in vivo cisplatin chemotherapy display elevated c-myc expression. Br J Cancer 73:610–614
Wood ZA, Schroder E, Robin Harris J, Poole LB (2003) Structure, mechanism and regulation of peroxiredoxins. Trends Biochem Sci 28:32–40
Zhou H, Kato A, Miyaji T, Yasuda H, Fujigaki Y, Yamamoto T, Yonemura K, Takebayashi S, Mineta H, Hishida A (2006) Urinary marker for oxidative stress in kidneys in cisplatin-induced acute renal failure in rats. Nephrol Dial Transplant 21:616–623
Acknowledgments
The first two authors (K.O. and D.M.) contributed equally to this work. This research was supported by the Japan Society for the Promotion of Science, Grant-in-Aid for Scientific Research (B), and Grant-in-Aid for Challenging Exploratory Research (T.Y.). We would like to thank Prof. G.E, Mann whose comments and suggestions were of inestimable value for this study.
Conflict of interest
None.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Okada, K., Ma, D., Warabi, E. et al. Amelioration of cisplatin-induced nephrotoxicity in peroxiredoxin I-deficient mice. Cancer Chemother Pharmacol 71, 503–509 (2013). https://doi.org/10.1007/s00280-012-2046-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00280-012-2046-0