Abstract
Objective
The objective of this study was to evaluate the efficacy and toxicity of docetaxel and cisplatin combination chemotherapy in patients with metastatic esophageal cancer.
Methods
Patients with untreated metastatic squamous cell esophageal cancer, which was histologically proven with at least one measurable lesion, were eligible for the study. Docetaxel 70 mg/m2 and cisplatin 70 mg/m2 were intravenously given on day 1 of 21 days schedule.
Results
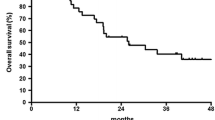
From December 2004 to December 2007, total of 39 patients (M/F = 39/0) were enrolled. The median age was 65 years. Thirty-four patients were evaluable for response. There were 3 (7.7%) complete remission, 10 (25.6%) partial remission, 11 (28.2%) stable disease, and 10 (25.6%) progression disease. The objective tumor response rate was 33.3% in intention-to-treat (ITT). Median PFS was 5.0 months and median survival was 8.3 months. Median number of cycles administered was 3. The relative dose intensity of docetaxel and cisplatin was 92 and 91%, respectively. This treatment was comparatively tolerated with grade 3/4 neutropenia in 20.5%/10.3%, grade 3 infection in 2.6% of patients.
Conclusion
Docetaxel plus cisplatin combination chemotherapy showed promising antitumor activity with manageable toxicities in patients with metastatic squamous esophageal cancer.

Similar content being viewed by others
References
Korean National Cancer Center (2007) Korean central cancer registry 22nd annual report. http://www.ncc.re.kr
Ajani JA, Ilson DH, Daugherty K et al (1994) Activity in patients with squamous cell carcinoma and adenocarcinoma of the esophagus. J Natl Cancer Inst 86:1086–1091
Coonley CJ, Bains M, Heelan R et al (1983) Phase II study of etoposide in the treatment of esophageal carcinoma. Cancer Treat Rep 67:397–398
Ajani JA (1994) Contribution of chemotherapy in the treatment of carcinoma of the esophagus: results and commentary. Semin Oncol 21:474–482
Polee MB, Eskens FA, van der Burg ME et al (2002) Phase II study of bi-weekly administration of paclitaxel and cisplatin in patients with advanced oesophageal cancer. Br J Cancer 86:669–673
Mauer A, Haraf D, Ferguson M et al (2000) Docetaxel-based combined modality therapy for locally advanced carcinoma of the esophagus and gastric cardia (abstract 954). Proc Ann Meet Am Soc Clin Oncol 19:246a
Einzig AL, Neuberg D, Remick SC et al (1996) Phase II trial of docetaxel (taxotere) in patients with adenocarcinoma of the upper gastrointestinal tract previously untreated with cytotoxic chemotherapy: the Eastern Cooperative Oncology group (ECOG) results of protocol E1293. Med Oncol 13:87–93
Heath EJ, Urba S, Marshall J et al (2002) Phase II trial of docetaxel chemotherapy in patient with incurable adenocarcinoma of the esophagus. Invest New Drugs 20:95–99
Woo IS, Jung KH, Park YL et al (1996) 5-Fluorouracil and cisplatin (FP) combination chemotherapy in advanced esophageal cancer. Korean Cancer Assoc 28:835–841
Bleiberg H, Conroy T, Paillot B et al (1997) Randomized phases II study of cisplatin and 5-fluorouracil (5-FU) versus cisplatin alone in advanced squamous cell esophageal cancer. Eur J Cancer 33:1216–1220
Sparreboom A, van Tellingen O, Nooijen WJ et al (1998) Preclinical pharmacokinetics of paclitaxel and docetaxel. Anticancer Drugs 9:1–17
Metges J, Hennequin C, Ychou M et al. (2001) Docetaxel as a second-line chemotherapy in metastatic esophageal cancer: a French study. Proc Am Soc Clin Oncol 20: 160a (Abstract 635)
Muro K, Hamaguchi T, Ohtsu A et al (2004) A phase II study of single-agent docetaxel in patients with metastatic esophageal cancer. Ann Oncol 15:955–959
Therasse P, Arbuck SG, Eisenhauer EA et al (2000) New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Nat Cancer Inst 92:205–216
Simon R (1989) Optimal two-stage designs for phase II clinical trials. Control Clin Trials 10:1–10
Long DL, Duffey PL, DeVita VT Jr et al (1991) The calculation of actual or received dose intensity; a comparison of published methods. J Clin Oncol 9:2042–2051
Jatoi A, Tirona MT, Cha SS et al (2002) A phase II trial of docetaxel and CPT-11 in patients with metastatic adenocarcinoma of the esophagus, gastroesophageal junction, and gastric cardia. Int J Gastrointest Cancer 32:115–123
Lordick F, von Schilling C, Bernhard H et al (2003) Phase II trial of irinotecn plus docetaxel in cisplatin-pretreated relapsed or refractory esophageal cancer. Br J Cancer 89:630–633
Petrasch S, Welt A, Reinacher A et al (1998) Chemotherapy with cisplatin and paclitaxel in patients with locally advanced, recurrent or metastatic esophageal cancer. Br J Cancer 78:511–514
Ilson DH, Ajani J, Ahalla K et al (1998) Phase II trial of paclitaxel, fluorouracil, and cisplatin in patients with advanced carcinoma of the esophagus. J Clin Oncol 16:1826–1834
Fosella FV, Lee JS, Murphy WK et al (1994) Phase II study of docetaxel for recurrent or metastatic non-small cell lung cancer. J Clin Oncol 12:1238–1244
Laack E, Andritzky B, Durk H et al (2005) Docetaxel and cisplatin as first-line treatment for patients with metastatic esophageal cancer: a pilot study. Onkologie 28:647–650
Lorenzen S, Duyster J, Lersch C et al (2005) Capecitabine plus docetaxel every 3 weeks in first- and second-line metastatic oesophageal cancer: final results of a phase II trial. Br J Cancer 92:2129–2133
Acknowledgments
Docetaxel (Taxotere) and study grant were provided by sanofi-aventis Korea.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kim, J.Y., Do, Y.R., Park, K.U. et al. A multi-center phase II study of docetaxel plus cisplatin as first-line therapy in patients with metastatic squamous cell esophageal cancer. Cancer Chemother Pharmacol 66, 31–36 (2010). https://doi.org/10.1007/s00280-009-1130-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00280-009-1130-6