Abstract
Autologous fat grafting is commonly performed in reconstructive breast surgery as well as in aesthetic breast augmentation surgery. Nevertheless, little is known about the interaction between fat grafts and cancer. A 36-year-old patient had undergone bilateral breast augmentation with autologous fat grafting. Two months after surgery, she perceived two small palpable indurations in the right breast. Nine months after the procedure, the lumps grew bigger and lumpectomy was performed. Histologic examination of the specimens showed mucinous carcinoma of the breast. This case raises once again the question about the possible links between breast cancer and fat grafts. The level of evidence is level V.
No Level assigned
This journal requires that authors assign a level of evidence to each article. For a full description of these Evidence-Based Medicine ratings, please refer to the Table of Contents or the online Instructions to Authors www.springer.com/00266.
Similar content being viewed by others
Introduction
Autologous fat grafting to the breast is a hot topic in plastic surgery currently, not only in breast reconstruction but also in cosmetic breast augmentation [1, 2]. Remarkable, long-lasting, natural improvements of breast size and shape can be achieved after this surgery [3]. But there are still unidentified concerns about the safety of breast fat grafting, mainly regarding cancer risks [4, 5]. This article describes a case of a 36-year-old woman with mucinous carcinoma in the right breast discovered 2 months after fat grafting.
Case Presentation
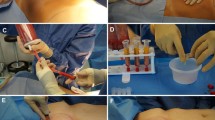
A 36-year-old woman received bilateral fat grafting to the breasts (400 ml per breast) (Fig. 1). Prior to the operation, magnetic resonance imaging (MRI) baseline imaging was performed to exclude any abnormality in the breasts. Fat was aspirated from the thighs and was washed and condensed before injection. Two months after the procedure, the patient perceived 2 small palpable indurations in the right breast. On mammography and ultrasound 3 months after the surgery, masses were detected in the subcutaneous layer (Fig. 2). She revisited our facility 9 months after the surgery complaining that the growing lumps were palpable and visible. Physical examination revealed 2 solitary elliptical movable masses in the inferiomedial quadrant of the right breast, measuring 4.5 and 3.5 cm in diameter, respectively. Slight pain was reported against pressure. No significant redness, infection, orange peel, malposition of the nipple, or abnormal discharge were observed. The axillary lymph node could not be palpated. There were no lumps in the left breast or axilla. Systemic examination did not reveal any significant abnormality. On diagnostic imaging, MRI indicated masses with low signal intensity on T1-weighted images and high signal intensity on T2-weighted images (Fig. 2). The subcutaneous masses were removed surgically by intraareolar approach. The excised specimens were grey white with a translucent, mucoid appearance. On gross, the lumps had complete capsules with massive adhesion to the surrounding tissue. Histologic examination of the specimens showed proliferation of neoplastic cells, new vessels and abundant mucus and was diagnosed as mucinous carcinoma (Fig. 3).
Above Gross appearance of the lump. The two lumps were elliptical in shape with complete capsules and massive adhesion to the surrounding tissue. The lumps were gray white with a translucent, mucoid appearance. Below Photomicrograph view of the lumps showing proliferation of neoplastic cells, new vessels, and abundant mucus. Hemalaun-eosin staining magnification: ×10 (left) and ×20 (right)
Discussion
Autologous fat injection into the breast for reconstruction or augmentation purposes has been widely performed recently. Complications include oil cysts, fat necrosis, and calcifications, but the main concern among surgeons is its safety regarding the oncological aspects [6]. In 2008, the French Society of Plastic, Reconstructive and Aesthetic Surgery issued a recommendation to postpone fat grafting to the breast in consideration of oncological risks [7]. To date, no informed consent can be given to patients stating that lipofilling does not stimulate dormant cancer cells or eventually induce new cancer cells [8]. For this patient, a causal link cannot be concluded between autologous fat grafting and cancer; however, it seems essential to discuss several points.
A lot of studies have reported the possible existence of an interaction between cancer and lipofilling to breast from the bench to the clinic. According to the studies of Petti and other researchers, the number of local events in the contralateral breast does not differ between the study group and control group, which indicates that lipofilling does not have a systemic effect [9]. Locally, the role of adipose tissue on tumor cells has been questioned for several reasons. On the one hand, mature adipocytes have been identified to be highly active endocrine cells secreting inflammatory cytokines, growth factors, and extracellular matrix (ECM). Of which, interleukin 6 (IL-6) plays the role of stimulating the invasive behavior of breast cancer cells, and ECM is capable of affecting tumor behavior to be more aggressive and metastasized. On the other hand, in addition to the functions of secreting inflammatory cytokines and growth factors, as the one of the most important preadipocytes in lipoaspirate [8], the role of ADSCs deserves to be questioned. It has been demonstrated in vitro and in animal models that ADSCs could increase migration and metastasis of breast cancer cells by increasing angiogenesis, decreasing local inflammation, and activating receptor site stem cells [10–15]. However, all of the conclusions come from the laboratory, clinical data show no significance in cancer occurrence rates between lipofilling groups and control groups except when the patients all had intraepithelial neoplasia [9, 16]. Considering the occurrence rate of breast cancer, it is of significance to mention that literally one out of 10 females will develop breast cancer during their lifetime, but the incidence is 0.1 % for patients who undergo fat grafting according to existing studies. According to Petit [9], cancer recurrence happened mostly after the third and fourth year after surgery and most of the existing literature does not report such a long mean follow-up period. In addition, the quality of most studies using GRADE is very low or low and the conclusion needs further confirmation by studies with a high level of evidence to further illustrate whether lipofilling could promote tumor cells to proliferate, differentiate, or metastasize or even induce de novo carcinogenesis.
In the case mentioned above, the possibility of inducing de novo carcinogenesis could not be denied. According to the literature, the time that carcinoma in situ develops into invasive carcinoma is 3–5 years. But in the case mentioned above, the tumor was discovered 2 months after the surgery. It is possible that the cancer was just a less than 5-mm diameter carcinoma in situ which cannot be discovered by MRI at the time of surgery. The addition of exoteric stimulus as well as the promoting functions of adipose tissue may have facilitated tumor cell proliferate. Besides, the injection movements could have disseminated the tumor in the breast and participated in the progress of cancer metastasis [17]. All patients undergoing autologous fat grafting should be followed up constantly and special attention should be paid to radiological follow-up [18]. For the previously reported two patients developing breast cancer with no delay, timely screening plays a significant role [9]. In this case, mammography and ultrasound are not so persuasive and sufficient as MRI in distinguishing benign and malignant lesions. Any patient identified with breast cancer, regardless of whether it is a new occurrence and recurrence, it should be recorded and shared worldwide. Further, biopsies may be performed if needed for additional clarification [19, 20].
Conclusions
This unusual case raises the question once again about the relations of lipofilling with breast cancer. As there is no strong scientific evidence in the literature, we suggest a multicenter controlled study on autologous fat grafting to identify or exclude the possibility with careful breast cancer surveillance. In addition, patients who received lipofilling should be carefully monitored over the long term systematically to ensure that the procedures do not mask early detection of cancer.
References
Delay E, Garson S, Tousson G, Sinna R (2009) Fat injection to the breast: technique, results, and indications based on 880 procedures over 10 years. Aesthet Surg J 29:360–376
Rigotti G, Marchi A, Stringhini P, Baroni G, Galiè M, Molino AM, Mercanti A, Micciolo R, Sbarbati A (2010) Determining the oncological risk of autologous lipoaspirate grafting for post-mastectomy breast reconstruction. Aesthet Plast Surg 34:475–480
Mu DL, Luan J, Mu L, Xin MQ (2009) Breast augmentation by autologous fat injection grafting: management and clinical analysis of complications. Ann Plast Surg 63:124–127
Largo RD, Tchang LA, Mele V, Scherberich A, Harder Y, Wettstein R, Schaefer DJ (2014) Efficacy, safety and complications of autologous fat grafting to healthy breast tissue: a systematic review. J Plast Reconstr Aesthet Surg 67:437–448
Coleman SR, Saboeiro AP (2007) Fat grafting to the breast revisited: safety and efficacy. Plast Reconstr Surg 119:775–785 discussion 786–787
Kling RE, Mehrara BJ, Pusic AL, Young VL, Hume KM, Crotty CA, Rubin JP (2013) Trends in autologous fat grafting to the breast: a national survey of the american society of plastic surgeons. Plast Reconstr Surg 132:35–46
Krastev TK, Jonasse Y, Kon M (2013) Oncological safety of autologous lipoaspirate grafting in breast cancer patients: a systematic review. Ann Surg Oncol 20:111–119
Wang YY, Ren GS, Petit JY, Muller C (2013) Oncological risk after autologous lipoaspirate grafting in breast cancer patients: from the bench to the clinic and back. J Craniofac Surg 24:700–702
Petit JY, Rietjens M, Botteri E, Rotmensz N, Bertolini F, Curigliano G, Rey P, Garusi C, De Lorenzi F, Martella S, Manconi A, Barbieri B, Veronesi P, Intra M, Brambullo T, Gottardi A, Sommario M, Lomeo G, Iera M, Giovinazzo V, Lohsiriwat V (2013) Evaluation of fat grafting safety in patients with intraepithelial neoplasia: a matched-cohort study. Ann Oncol 24:1479–1484
Rowan BG, Gimble JM, Sheng M, Anbalagan M, Jones RK, Frazier TP, Asher M, Lacayo EA, Friedlander PL, Kutner R, Chiu ES (2014) Human adipose tissue-derived stromal/stem cells promote migration and early metastasis of triple negative breast cancer xenografts. PLoS One 9:e89595
Crisostomo PR, Markel TA, Wang Y, Meldrum DR (2008) Surgically relevant aspects of stem cell paracrine effects. Surgery 143:577–581
Martin-Padura I, Gregato G, Marighetti P, Mancuso P, Calleri A, Corsini C, Pruneri G, Manzotti M, Lohsiriwat V, Rietjens M, Petit JY, Bertolini F (2012) The white adipose tissue used in lipotransfer procedures is a rich reservoir of CD34+ progenitors able to promote cancer progression. Cancer Res 72:325–334
Schaffler A, Scholmerich J, Buechler C (2007) Mechanisms of disease: adipokines and breast cancer—endocrine and paracrine mechanisms that connect adiposity and breast cancer. Nat Clin Pract Endocrinol Metab 3(4):345–354
Dieudonne MN, Bussiere M, Dos SE, Leneveu MC, Giudicelli Y, Pecquery R (2006) Adiponectin mediates antiproliferative and apoptotic responses in human MCF7 breast cancer cells. Biochem Biophys Res Commun 345:271–279
Lohsiriwat V, Curigliano G, Rietjens M, Goldhirsch A, Petit JY (2011) Autologous fat transplantation in patients with breast cancer: “silencing” or “fueling” cancer recurrence. Breast. 20:351–357
Delay E, Garson S, Tousson G, Sinna R (2009) Fat injection to the breast: technique, results, and indications based on 880 procedures over 10 years. Aesthet Surg J 29:360–376
Alharbi M, Garrido I, Vaysse C, Chavoin JP, Grolleau JL, Chaput B (2013) Latissimus dorsi flap invasion by ductal breast carcinoma after lipofilling. Plast Reconstr Surg Glob Open. 1:e68
Pearl RA, Leedham SJ, Pacifico MD (2012) The safety of autologous fat transfer in breast cancer: lessons from stem cell biology. J Plast Reconstr Aesthet Surg 65:283–288
Fiaschetti V, Pistolese CA, Fornari M, Liberto V, Cama V, Gentile P, Floris M, Floris R, Cervelli V, Simonetti G (2013) Magnetic resonance imaging and ultrasound evaluation after breast autologous fat grafting combined with platelet-rich plasma. Plast Reconstr Surg 132:498e–509e
Vallejo A, Urban C, Zucca-Matthes G, Rietjens M (2013) Is there enough evidence to use lipofilling in breast cancer reconstruction. Plast Reconstr Surg 132:689e–691e
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors have no financial interest to declare in relation to the content of this article.
Rights and permissions
About this article
Cite this article
Cheng, L., Han, XF., Zhang, C. et al. Occurrence of Breast Mucinous Carcinoma After Autologous Fat Grating for Breast Augmentation. Aesth Plast Surg 40, 102–105 (2016). https://doi.org/10.1007/s00266-015-0605-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00266-015-0605-6