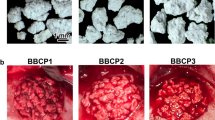
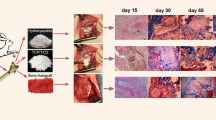
Abstract
Purpose: Hydroxyapatite/tri-calcium phosphate (HA/TCP) mixture is an osteoconductive material used as a bone graft substitute, and demineralised bone matrix (DBM) is an osteoinductive material. A combination of DBM and HA/TCP mixture would probably create a composite with both osteoconductive and osteoinductive properties. The purpose of this study was to determine the effect of the combination of DBM and HA/TCP mixture on healing of rat radius segmental defects. Methods: Twenty-four adult male Wistar rats were used. Bilateral radial defects were created in each animal. Radial defects were implanted with DBM, HA/TCP mixture and a combination of both substances. Control defects were left unfilled. Ten weeks after implantation, the animals were sacrificed, and the radii were evaluated by radiograhic and histopathological studies. Results: The use of DBM alone demonstrated improved healing on radiographic and histological studies compared to other groups and the control group. There were no differences between the other two groups and the control group. Conclusion: The DBM group showed the best healing response. Combined use of DBM and HA/TCP mixture did not improve bone healing, and the osteoinductive properties of DBM were inhibited by HA/TCP mixture.
Résumé
Le mélange hydroxyapatite/phosphate tri-calcique (HA/TCP) est un matériel d’ostéo conduction utilisé comme greffe ou substitut osseux comme les matrices osseuses déminéralisées (DBM) utilisées en tant que matériel d’ostéo induction. La combinaison de DBM et HA/TCP devrait probablement avoir les deux propriétés d’ostéo induction et d’ostéo conduction. Le propos de cette étude est d’évaluer les effets d’un mélange de DBM et d’HA/TCP sur la consolidation de défauts osseux du radius chez le rat. Vingt quatre rats adultes Wistar ont été utilisés. Chaque animal était préparé avec un defect radial bilatéral. Les radius droits des rats ont été implantés soit avec DBM, soit avec HA/TCP isolée, soit avec une combinaison des deux substances, le radius gauche servant de contrôle. Dix semaines après l’implantation, les animaux ont été sacrifiés et les os ont été évalués sur la plan radiographique et histopathologique. L’utilisation de DBM isolée montre une amélioration de la guérison sur le plan radiographique et histologique. On ne retrouve pas de différence avec les autres groupes. En conclusion, le groupe DBM montre une bonne réponse sur le plan de la consolidation. L’utilisation d’une mixture DBM et HA/TCP n’entraîne pas une amélioration et une guérison, les propriétés d’ostéo induction du DBM étant inhibées par le mélange HA/TCP.





Similar content being viewed by others
Change history
17 December 2018
The first name of one author is missing in the published article. The correct abbreviation: H.C. Gemalmaz.
17 December 2018
The first name of one author is missing in the published article. The correct abbreviation: H.C. Gemalmaz.
17 December 2018
The first name of one author is missing in the published article. The correct abbreviation: H.C. Gemalmaz.
17 December 2018
The first name of one author is missing in the published article. The correct abbreviation: H.C. Gemalmaz.
References
Alper G, Bernick S, Yazdi M, Nimni ME (1989) Osteogenesis in bone defects in rats: the effects of hydroxyapatite and demineralized bone matrix. Am J Med Sci 298(6):371–376
Bauer TW, Muschler GF (2000) Bone graft materials. An overview of the basic science. Clin Orthop 371:10–27
Bingel SA (1999) Euthanasia and necropsy. In: An YH, Friedman RJ (eds) Animal models in orthopaedic research. CRC Press, Boca Raton, pp 71–81
Burchardt H (1987) Biology of bone transplantation. Orthop Clin North Am 18:187–196
Cook SD, Baffes GC, Wolfe MW, Sampath TK, Rueger DC (1994) Recombinant human bone morphogenetic protein-7 induces healing in a canine long-bone segmental defect model. Clin Orthop 301:302–312
Farso-Nielsen F, Karring T, Gogolewski S (1992) Biodegradable guide for bone regeneration. Polyurethane membranes tested in rabbit radius defects. Acta Orthop Scand 63:66–69
Finkemeier CG (2002) Bone-grafting and bone-graft substitutes. J Bone Joint Surg Am 84(3):454–464
Fleming JE, Cornell CN, Muschler GF (2000) Bone cells and matrices in orthopedic tissue engineering. Orthop Clin North Am 31:357–374
Gepstein R, Weiss RE, Hallel T (1987) Bridging large defects in bone by demineralized bone matrix in the form of a powder. A radiographic, histological, and radioisotope-uptake study in rats. J Bone Joint Surg Am 69:984–992
Greenwald AS, Boden SD, Goldberg VM, Khan Y, Laurencin CT, Rosier RN (2001) American Academy of Orthopaedic Surgeons. The Committee on Biological Implants. Bone-graft substitutes: facts, fictions, and applications. J Bone Joint Surg Am 83 Suppl 2 Pt 2:98–103
Gruber HE, Stasky AA (1999) Histologic study in orthopaedic animal research. In: An YH, Friedman RJ (eds) Animal models in orthopaedic research. CRC Press, Boca Raton, pp 115–137
Hopp SG, Dahners LE, Gilbert JA (1989) A study of the mechanical strength of long bone defects treated with various bone autograft substitutes: an experimental investigation in the rabbit. J Orthop Res 7(4):579–584
Lane JM, Sandhu HS (1987) Current approaches to experimental bone grafting. Orthop Clin North Am 18:213–225
Moore DC, Chapman MW, Manske D (1987) The evaluation of a biphasic calcium phosphate ceramic for use in grafting long-bone diaphyseal defects. J Orthop Res 5(3):356–365
Nunamaker DM (1998) Experimental models of fracture repair. Clin Orthop 355(Suppl):S56–S65
Nyman R, Magnusson M, Sennerby L, Nyman S, Lundgren D (1995) Membrane-guided bone regeneration. Segmental radius defects studied in the rabbit. Acta Orthop Scand 66:169–173
Ozturk AM, Cila E, Kanatli U, Isik I, Senkoylu A, Uzunok D, Piskin E (2005) Treatment of segmental bone defects in rats by the stimulation of bone marrow osteo-progenitor cells with prostaglandin E(2). Int Orthop 29(2):73–77
Parikh SN (2002) Bone graft substitutes in modern orthopedics. Orthopedics 25(11):1301–1309
Ragni P, Ala-Mononen P, Lindholm TS (1993) Spinal fusion induced by porous hydroxyapatite blocks (HA). Experimental comparative study with HA, demineralized bone matrix and autogenous bone marrow. Ital J Orthop Traumatol 19(1):133–144
Ragni P, Lindholm TS (1991) Interaction of allogeneic demineralized bone matrix and porous hydroxyapatite bioceramics in lumbar interbody fusion in rabbits. Clin Orthop 272:292–299
Salkeld SL, Patron LP, Barrack RL, Cook SD (2001) The effect of osteogenic protein-1 n the healing of segmental one defects treated with utograft or allograft bone. J Bone Joint Surg Am 83(6):803–816
Solheim E, Pinholt EM, Andersen R, Bang G, Sudmann E (1992) The effect of a composite of polyorthoester and demineralized bone on the healing of large segmental defects of the radius in rats. J Bone Joint Surg Am 74:1456–1463
Thalgott JS, Giuffre JM, Klezl Z, Timlin M (2002) Anterior lumbar interbody fusion with titanium mesh cages, coralline hydroxyapatite, and demineralized bone matrix as part of a circumferential fusion. Spine 2(1):63–69
Whang PH, Wang JC (2003) Bone graft substitutes for spinal fusion. Spine 3(2):155–165
Wixson SK, Smiler KL (1998) Anesthesia and analgesia in rodents. In: Kohn DF, Wixson SK, White WJ, Benson GJ (eds) Anesthesia and analgesia in laboratory animals. Academic, San Diego, pp 173–184
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Öztürk, A., Yetkin, H., Memis, L. et al. Demineralized bone matrix and hydroxyapatite/tri-calcium phosphate mixture for bone healing in rats. International Orthopaedics (SICO 30, 147–152 (2006). https://doi.org/10.1007/s00264-006-0079-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00264-006-0079-x