Abstract
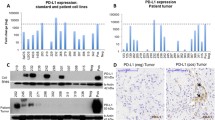
Ependymomas are biologically and clinically heterogeneous tumors of the central nervous system that have variable clinical outcomes. The status of the tumor immune microenvironment in ependymoma remains unclear. Immune cell subsets and programmed death ligand 1 (PD-L1) expression were measured in 178 classical ependymoma cases by immunohistochemistry using monoclonal antibodies that recognized tumor-infiltrating lymphocyte subsets (TILs; CD3, CD4, CD8, FOXP3, and CD20), tumor-associated macrophages (TAMs; CD68, CD163, AIF1), indoleamine 2,3-dioxygenase (IDO)+ cells and PD-L1-expressing tumor cells. Increases in CD3+ and CD8+ cell numbers were associated with a prolonged PFS. In contrast, increased numbers of FOXP3+ and CD68+ cells and a ratio of CD163/AIF1+ cells were significantly associated with a shorter PFS. An increase in the IDO+ cell number was associated with a significantly longer PFS. To consider the quantities of TILs, TAMs, and IDO+ cells together, the cases were clustered into 2 immune cell subgroups using a k-means clustering analysis. Immune cell subgroup A, which was defined by high CD3+, low CD68+ and high IDO+ cell counts, predicted a favorable PFS compared to subgroup B by univariate and multivariate analyses. We found six ependymoma cases expressing PD-L1. All these cases were supratentorial ependymoma, RELA fusion-positive (ST-RELA). PD-L1 expression showed no prognostic significance. This study showed that the analysis of tumor-infiltrating immune cells could aid in predicting the prognosis of ependymoma patients and in determining therapeutic strategies to target the tumor microenvironment. PD-L1 expression in the ST-RELA subgroup suggests that this marker has a potential added value for future immunotherapy treatments.




Similar content being viewed by others
Abbreviations
- AIF1:
-
Allograft inflammatory factor 1
- CNS:
-
Central nervous system
- GTS:
-
Gross total resection
- ICC:
-
Intraclass correlation coefficient
- KPS:
-
Karnofsky Performance Scale
- NF2:
-
Neurofibromatosis type 2
- PF:
-
Posterior fossa
- PF-A:
-
Posterior fossa ependymoma, group A
- PF-B:
-
Posterior fossa ependymoma, group B
- SP:
-
Spinal cord
- ST:
-
Supratentorial
- ST-RELA:
-
Supratentorial ependymoma, RELA fusion-positive
- STR:
-
Subtotal resection
- TAMs:
-
Tumor-associated macrophages/microglia
References
Kim YJ, Kim JY, Lim do H et al (2013) Retrospective analysis of treatment outcome of pediatric ependymomas in Korea: analysis of Korean multi-institutional data. J Neurooncol 113:39–48. https://doi.org/10.1007/s11060-013-1087-5
Nuno M, Yu JJ, Varshneya K et al (2016) Treatment and survival of supratentorial and posterior fossa ependymomas in adults. J Clin Neurosci 28:24–30. https://doi.org/10.1016/j.jocn.2015.11.014
Yang T, Wu L, Yang C, Deng X, Xu Y (2014) Clinical features and long-term outcomes of intraspinal ependymomas in pediatric patients. Child’s Nerv Syst ChNS 30:2073–2081. https://doi.org/10.1007/s00381-014-2528-y
Louis DN, Ohgaki H, Wiestler OD, Cavenee WK (2016) WHO Classification of Tumours of the Central Nervous System, Revised. Fourth Edition. IARC WHO Classification of Tumours
Pajtler KW, Witt H, Sill M et al (2015) Molecular classification of ependymal tumors across all CNS compartments, histopathological grades, and age groups. Cancer Cell 27:728–743. https://doi.org/10.1016/j.ccell.2015.04.002
Xue S, Hu M, Iyer V, Yu J (2017) Blocking the PD-1/PD-L1 pathway in glioma: a potential new treatment strategy. J Hematol Oncol 10:81. https://doi.org/10.1186/s13045-017-0455-6
Srinivasan VM, Ferguson SD, Lee S et al (2017) Tumor vaccines for malignant gliomas. Neurotherapeutics 14:345–357. https://doi.org/10.1007/s13311-017-0522-2
Swartz MA, Iida N, Roberts EW et al (2012) Tumor microenvironment complexity: emerging roles in cancer therapy. Cancer Res 72:2473–2480. https://doi.org/10.1158/0008-5472.CAN-12-0122
Witt DA, Donson AM, Amani V et al (2018) Specific expression of PD-L1 in RELA-fusion supratentorial ependymoma: Implications for PD-1-targeted therapy. Pediatr Blood Cancer 65:e26960. https://doi.org/10.1002/pbc.26960
Kerkar SP, Restifo NP (2012) Cellular constituents of immune escape within the tumor microenvironment. Cancer Res 72:3125–3130. https://doi.org/10.1158/0008-5472.CAN-11-4094
Han S, Zhang C, Li Q et al (2014) Tumour-infiltrating CD4(+) and CD8(+) lymphocytes as predictors of clinical outcome in glioma. Br J Cancer 110:2560–2568. https://doi.org/10.1038/bjc.2014.162
Kmiecik J, Poli A, Brons NH et al (2013) Elevated CD3+ and CD8+ tumor-infiltrating immune cells correlate with prolonged survival in glioblastoma patients despite integrated immunosuppressive mechanisms in the tumor microenvironment and at the systemic level. J Neuroimmunol 264:71–83. https://doi.org/10.1016/j.jneuroim.2013.08.013
Sayour EJ, McLendon P, McLendon R et al (2015) Increased proportion of FoxP3+ regulatory T cells in tumor infiltrating lymphocytes is associated with tumor recurrence and reduced survival in patients with glioblastoma. Cancer Immunol Immunother 64:419–427. https://doi.org/10.1007/s00262-014-1651-7)
Yue Q, Zhang X, Ye H-x et al (2014) The prognostic value of Foxp3+ tumor-infiltrating lymphocytes in patients with glioblastoma. J Neurooncol 116:251–259. https://doi.org/10.1007/s11060-013-1314-0)
Domingues P, Gonzalez-Tablas M, Otero A et al (2016) Tumor infiltrating immune cells in gliomas and meningiomas. Brain Behav Immun 53:1–15. https://doi.org/10.1016/j.bbi.2015.07.019
Kennedy BC, Showers CR, Anderson DE et al (2013) Tumor-associated macrophages in glioma: friend or foe? J Oncol 2013:486912. https://doi.org/10.1155/2013/486912
Ye XZ, Xu SL, Xin YH et al (2012) Tumor-associated microglia/macrophages enhance the invasion of glioma stem-like cells via TGF-beta1 signaling pathway. J Immunol 189:444–453. https://doi.org/10.4049/jimmunol.1103248
Munn DH, Mellor AL (2013) Indoleamine 2,3 dioxygenase and metabolic control of immune responses. Trends Immunol 34:137–143. https://doi.org/10.1016/j.it.2012.10.001
Wilke CM, Zou W (2011) T lymphocytes to IDO+ cells: check. Blood 117:2082–2083. https://doi.org/10.1182/blood-2010-12-322172
Godin-Ethier J, Hanafi LA, Piccirillo CA, Lapointe R (2011) Indoleamine 2,3-dioxygenase expression in human cancers: clinical and immunologic perspectives. Clin Cancer Res 17:6985–6991. https://doi.org/10.1158/1078-0432.CCR-11-1331
Zhai L, Ladomersky E, Lauing KL et al (2017) Infiltrating T cells increase ido1 expression in glioblastoma and contribute to decreased patient survival. Clin Cancer Res 23:6650–6660. https://doi.org/10.1158/1078-0432.CCR-17-0120
Wainwright DA, Chang AL, Dey M et al (2014) Durable therapeutic efficacy utilizing combinatorial blockade against IDO, CTLA-4, and PD-L1 in mice with brain tumors. Clin Cancer Res 20:5290–5301. https://doi.org/10.1158/1078-0432.CCR-14-0514
Wainwright DA, Balyasnikova IV, Chang AL et al (2012) IDO expression in brain tumors increases the recruitment of regulatory T cells and negatively impacts survival. Clin Cancer Res 18:6110–6121. https://doi.org/10.1158/1078-0432.CCR-12-2130
Wainwright DA, Dey M, Chang A, Lesniak MS (2013) Targeting tregs in malignant brain cancer: overcoming IDO. Front Immunol 4:116. https://doi.org/10.3389/fimmu.2013.00116
Donson AM, Birks DK, Barton VN et al (2009) Immune gene and cell enrichment is associated with a good prognosis in ependymoma. J Immunol 183:7428–7440. https://doi.org/10.4049/jimmunol.0902811
Nam SJ, Go H, Paik JH et al (2014) An increase of M2 macrophages predicts poor prognosis in patients with diffuse large B-cell lymphoma treated with rituximab, cyclophosphamide, doxorubicin, vincristine and prednisone. Leukemia Lymphoma 55:2466–2476. https://doi.org/10.3109/10428194.2013.879713
Budczies J, Klauschen F, Sinn BV et al (2012) Cutoff Finder: a comprehensive and straightforward web application enabling rapid biomarker cutoff optimization. PLoS One 7:e51862. https://doi.org/10.1371/journal.pone.0051862
Patil PA, Blakely AM, Lombardo KA et al (2018) Expression of PD-L1, indoleamine 2,3-dioxygenase and the immune microenvironment in gastric adenocarcinoma. Histopathology. https://doi.org/10.1111/his.13504 (epub ahead of print)
Parker M, Mohankumar KM, Punchihewa C et al (2014) C11orf95-RELA fusions drive oncogenic NF-kappaB signalling in ependymoma. Nature 506:451–455. https://doi.org/10.1038/nature13109
Witt H, Mack SC, Ryzhova M et al (2011) Delineation of two clinically and molecularly distinct subgroups of posterior fossa ependymoma. Cancer Cell 20:143–157. https://doi.org/10.1016/j.ccr.2011.07.007
Mack SC, Witt H, Piro RM et al (2014) Epigenomic alterations define lethal CIMP-positive ependymomas of infancy. Nature 506:445–450. https://doi.org/10.1038/nature13108
Gupta K, Salunke P (2015) Understanding ependymoma oncogenesis: an update on recent molecular advances and current perspectives. Mol Neurobiol 54:15–21. https://doi.org/10.1007/s12035-015-9646-8
Pajtler KW, Mack SC, Ramaswamy V et al (2017) The current consensus on the clinical management of intracranial ependymoma and its distinct molecular variants. Acta Neuropathol 133:5–12. https://doi.org/10.1007/s00401-016-1643-0
Thompson YY, Ramaswamy V, Diamandis P, Daniels C, Taylor MD (2015) Posterior fossa ependymoma: current insights. Child’s Nerv Syst ChNS. 31:1699–1706. https://doi.org/10.1007/s00381-015-2823-2
Wani K, Armstrong TS, Vera-Bolanos E et al (2012) A prognostic gene expression signature in infratentorial ependymoma. Acta Neuropathol 123:727–738. https://doi.org/10.1007/s00401-012-0941-4
Archer TC, Pomeroy SL (2011) Posterior fossa ependymomas: a tale of two subtypes. Cancer Cell 20:133–134. https://doi.org/10.1016/j.ccr.2011.08.003
Ebert C, von Haken M, Meyer-Puttlitz B et al (1999) Molecular genetic analysis of ependymal tumors. Am J Pathol 155:627–632. https://doi.org/10.1016/s0002-9440(10)65158-9
Korshunov A, Neben K, Wrobel G et al (2003) Gene expression patterns in ependymomas correlate with tumor location, grade, and patient age. Am J Pathol 163:1721–1727. https://doi.org/10.1016/s0002-9440(10)63530-4
Griesinger AM, Josephson RJ, Donson AM et al (2015) Interleukin-6/STAT3 pathway signaling drives an inflammatory phenotype in group A ependymoma. Cancer Immunol Res 3:1165–1174. https://doi.org/10.1158/2326-6066.CIR-15-0061
Gousias K, Markou M, Arzoglou V et al (2010) Frequent abnormalities of the immune system in gliomas and correlation with the WHO grading system of malignancy. J Neuroimmunol 226:136–142. https://doi.org/10.1016/j.jneuroim.2010.05.027
Sanmamed MF, Chen L (2014) Inducible expression of B7-H1 (PD-L1) and its selective role in tumor site immune modulation. Cancer J 20:256–261. https://doi.org/10.1097/PPO.0000000000000061
Wei F, Zhong S, Mae Z et al (2013) Strength of PD-1 signaling differentially affects T-cell effector functions. Proc Natl Acad Sci USA 110:10892. https://doi.org/10.1073/pnas.1305394110
Ellison DW, Kocak M, Figarella-Branger D et al (2011) Histopathological grading of pediatric ependymoma: reproducibility and clinical relevance in European trial cohorts. J Negat Results Biomed 10:7. https://doi.org/10.1186/1477-5751-10-7
Author information
Authors and Affiliations
Contributions
Study conception and design were performed by SJN and COS. SJN, COS, and SKK reviewed the pathological materials according to current WHO criteria. SJN, Y-HK, and JEP reviewed and obtained detailed clinical data. Y-sR organized cohort of pediatric ependymoma patients and obtained clinical data from the medical records. YHC, and JHK organized cohort of adult ependymoma patients and obtained clinical data from the medical records. Statistical analysis was performed by SJN and COS. SJN prepared the initial manuscript. All co-authors made substantial contributions to the rewriting of the manuscript, review, and approval.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval and ethical standards.
This study was performed with archived paraffin-embedded tissue samples. This study was approved by the Asan Medical Center Institutional review board (approval number 2016 − 1197) and was conducted in accordance with the Declaration of Helsinki.\
Informed consent
Informed consent by individual patients could not be given, as the study only included paraffin-embedded archived tissue. With the approval of the ethical committee, informed consent was not required because all patient data were anonymized.
Additional information
Soo Jeong Nam and Chang Ohk Sung are corresponding authors.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Nam, S.J., Kim, YH., Park, J.E. et al. Tumor-infiltrating immune cell subpopulations and programmed death ligand 1 (PD-L1) expression associated with clinicopathological and prognostic parameters in ependymoma. Cancer Immunol Immunother 68, 305–318 (2019). https://doi.org/10.1007/s00262-018-2278-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00262-018-2278-x