Abstract
Objective
IRX-2, a primary cell-derived biologic with pleotropic immune activity, was shown to induce increased lymphocyte infiltrations into the tumor of patients with head and neck squamous cell cancer (HNSCC) after 10 days of neoadjuvant therapy (Berinstein et al. 2011). In the same patients enrolled in the Phase II study, peripheral blood lymphocyte subsets were monitored pre- and post-IRX-2 therapy to evaluate changes induced by IRX-2.
Methods
Absolute lymphocyte numbers were determined in whole blood using the TetraONE System. Lymphocytes were further separated on Ficoll—Hypaque gradients and evaluated by multiparameter flow cytometry. Lymphocyte numbers, including regulatory T cells (Treg) and naïve, memory and effector T cells, were compared in pre- and post-therapy specimens.
Results
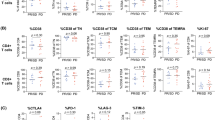
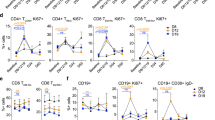
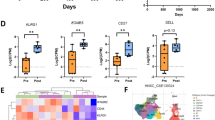
Total lymphocyte numbers remained unchanged after IRX-2 therapy. Significant changes occurred in numbers of circulating B cells and NKT cells, which decreased following IRX-2 therapy. The frequency of circulating Treg (CD4+CD25high) remained unaltered (e.g., 6.7 ± 0.6% vs. 7.5 ± 0.8%; means ± SEM) as was the CD8+/Treg ratio (6.6 before and 6.7 after IRX-2 therapy). The mean absolute number of CD3+CD45RA+CCR7+ (naïve) T cells was decreased after IRX-2 therapy but numbers of total memory (i.e., central and peripheral) and terminally differentiated T cells were unchanged.
Conclusions
IRX-2-mediated reductions in B and NKT cell numbers in the blood suggest a redistribution of these cells to tissues. A decrease in naïve T cells implies their up-regulated differentiation to memory T cells. Unchanged Treg numbers after IRX-2 therapy indicate that IRX-2 does not expand this compartment, potentially benefiting anti-tumor immune responses.


Similar content being viewed by others
Notes
Berinstein et al. [26].
References
Whiteside TL (2005) Immunobiology of head and neck cancer. Cancer Metastasis Rev 24:95–105
Specenier P, Vermorken JB (2011) Cetuximab in the treatment of squmaous cell carcinoma of the head and neck. Exp Rev Anticancer Ther 11:511–524
Janke M, Peeters B, Zhao H, deLeeuw O, Moorman R, Arnold A, Ziouta Y, Fournier P, Schirrmacher V (2008) Activation of human T cells by a tumor vaccine infected with recombinant newcastle disease virus producing IL-2. Int J Oncol 33:823–832
Ferris RL, Whiteside TL, Ferrone S (2006) Immune escape associated with functional defects in antigen-processing machinery in head and neck cancer. Clin Cancer Res 12:3890–3895
Young RM (2006) Protective mechanisms of head and neck squamous cell carcinomas from immune assult. Head Neck 28:462–470
Hadden JW (1995) The immunology of head and neck cancer: prospects for immunotherapy. Clin Immunother 3:362–385
Whiteside TL, Letessier E, Hirabayashi H, Vitolo D, Bryant J, Barnes L, Snyderman C, Johnson JT, Myers E, Herberman RB et al (1993) Evidence for local and systemic activation of immune cells by peritumoral injections of interleukin 2 in patients with advanced squamous cell carcinoma of the head and neck. Cancer Res 53:5654–5662
Cortesina G, De Stefani A, Giovarelli M, Barioglio MG, Cavallo GP, Jemma C, Forni G (1988) Treatment of recurrent squamous cell carcinoma of the head and neck with low doses of interleukin-2 injected perilymphatically. Cancer 62:2482–2485
Cortesina G, De Stefani A, Galeazzi E, Cavallo GP, Badellino F, Margarino G, Jemma C, Forni G (1994) Temporary regression of recurrent squamous cell carcinoma of the head and neck is achieved with a low but not with a high dose of recombinant interleukin-2 injected perilymphatically. Br J Cancer 69:572–576
De Stefani A, Valente G, Forni G, Lerda W, Ragona R, Cortesina G (1996) Treatment of oral cavity and oropharynx squamous cell carcinoma with perilymphatic interleukin-2: Clinical and pathologic correlations. J Immunother 19:125–133
Hadden JW, Endicott J, Baekey P, Skipper P, Hadden EM (1994) Interleukins and contrasuppression induce immune regression of head and neck cancer. Arch Otolaryngol Head Neck Surg 120:395–403
Meneses A, Verastegui E, Barrera JL, Zinser J, de la Garza J, Hadden JW (1998) Histologic findings in subjects with head and neck squamous cell carcinoma receiving perilymphatic natural cytokine mixture prior to surgery. Arch Pathol Lab Med 122:447–454
Freeman SM, Franco JL, Kenady DE, Baltzer L, Roth Z, Brandwein HJ, Hadden JW (2011) A Phase I safety study of an IRX-2 regimen in subjects with squamous cell carcinoma of the head and neck. Am J Clin Oncol 34:173–178
Wolf GT, Fee WE, Dolan RW, Moyer JS, Kaplan MJ, Spring PM, Suen J, Kenady DE, Newman JG, Carroll WR et al (2011) Novel neoadjuvant immunotherapy regimen safety and survival in head and neck squamous cell cancer. Head Neck. doi:10.1002/hed.21660
Berlinger NT (1984) Deficient immunity in head and neck cancer due to excessive monocyte production of prostaglandins. Laryngoscope 94:1407–1411
Hadden J (1995) The treatment of zinc deficiency as an immunotherapy. Int J Immunopharmac 17:697
Strauss L, Bergmann C, Gooding W, Johnson JT, Whiteside TL (2007) The frequency and suppressor function of CD4+ CD25highFoxp3+ T cells in the peripheral circulation of patients with squamous cell carcinoma of the head and neck. Clin Cancer Res 13:6301–6311
Kuss I, Hathaway B, Ferris RL, Gooding W, Whiteside TL (2004) Decreased absolute counts of T lymphocyte subsets and their relation to disease in squamous cell carcinoma of the head and neck. Clin Cancer Res 10:3755–3762
Pretscher D, Distel LV, Grabenbauer GG, Wittlinger M, Buettner M, Niedobitek G (2009) Distribution of immune cells in head and neck cancer: CD8+ T-cells and CD20+ B-cells in metastatic lymph nodes are associated with favourable outcome in patients with oro- and hypopharyngeal carcinoma. BMC Cancer 9:292–301
Hoffmann TK, Donnenberg AD, Finkelstein SD, Donnenberg VS, Friebe-Hoffman U, Myers EN, Appella E, DeLeo AB, Whiteside TL (2002) Frequencies of tetramer+ T cells specific for the wild-type sequence p53264–272 peptide in the circulations of patients with head and neck cancer. Cancer Res 62:3521–3529
Kim J-W, Ferris RL, Whiteside TL (2005) Chemokine receptor 7 (CCR7) expression and protection of circulating CD8+ T lymphocytes from apoptosis. Clin Cancer Res 11:7901–7910
Berntsen A, Brimnes MK, thor Straten P, Svane IM (2010) Increase of circulating CD4+CD25highFOXP3+ regulatory T cells in patients with metastatic renal cell carcinoma during treatment with dendritic cell vaccination and low-dose interleukin-2. J Immunother 33:425–434
Bergmann C, Strauss L, Wang Y, Szczepanski MJ, Lang S, Johnson JT, Whiteside TL (2008) T regulatory type 1 cells (Tr1) in squamous cell carcinoma of the head and neck: mechanisms of suppression and expansion in advanced disease. Clin Cancer Res 14:3706–3715
Bergmann C, Strauss L, Zeidler R, Lang S, Whiteside TL (2007) Expansion of human T regulatory type 1 cells in the microenvironment of COX-2 overexpressing head and neck squamous cell carcinoma. Cancer Res 67:8865–8873
Czystowska M, Strauss L, Bergmann C, Szajnik M, Rabinowich H, Whiteside TL (2010) Reciprocal granzyme/perforin-mediated death of human regulatory and responder T cells is regulated by interleukin-2 (IL-2). J Mol Med 88:577–588
Berinstein NL, Wolf GT, Naylor PH, Baltzer L, Egan JE, Brandwein HJ, Whiteside TL, Goldstein L, El-Naggar A, Badoual C, Fridman W-H, White JM, Hadden JW (2011) Increased lymphocyte infiltration in patients with head and neck cancer treated with the IRX-2 immunotherapy regimen. Cancer Immunol Immunother (in press)
Acknowledgments
Financial Support: Supported in part by a grant from NIH (PO1-CA109688) and by IRX Therapeutics Inc.
Conflict of interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Whiteside, T.L., Butterfield, L.H., Naylor, P.H. et al. A short course of neoadjuvant IRX-2 induces changes in peripheral blood lymphocyte subsets of patients with head and neck squamous cell carcinoma. Cancer Immunol Immunother 61, 783–788 (2012). https://doi.org/10.1007/s00262-011-1136-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00262-011-1136-x