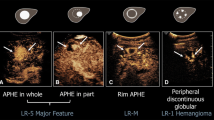
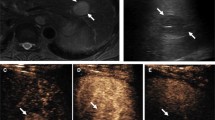
Abstract
Contrast-enhanced ultrasound (CEUS) is a specific form of ultrasound imaging performed with intravenous administration of microbubble contrast agents. It has been extensively used for liver tumor characterization and was recently added to the American College of Radiology Liver Imaging Reporting and Data System (CEUS LI-RADS). This paper describes technical recommendations for successful liver CEUS lesion characterization, and provides imaging protocol and Lexicon of imaging findings.
















Similar content being viewed by others
References
World Health Organization (2012) GLOBOCAN 2012: estimated cancer incidence, mortality and prevalence worldwide in 2017. http://globocan.iarc.fr/Pages/fact_sheets_cancer.aspx
Mitchell DG, Bruix J, Sherman M, Sirlin CB (2015) LI-RADS (liver imaging reporting and data system): summary, discussion, and consensus of the LI-RADS management working group and future directions. Hepatology. 61:1056–1065
Heimbach J, Kulik LM, Finn R, et al. (2017) AASLD guidelines for the treatment of hepatocellular carcinoma. Hepatology. https://doi.org/10.1002/hep.29086
Vilana R, Forner A, Bianchi L, et al. (2010) Intrahepatic peripheral cholangiocarcinoma in cirrhosis patients may display a vascular pattern similar to hepatocellular carcinoma on contrast-enhanced ultrasound. Hepatology 51(6):2020–2029
Li R, Yuan MX, Ma KS, et al. (2014) Detailed analysis of temporal features on contrast enhanced ultrasound may help differentiate intrahepatic cholangiocarcinoma from hepatocellular carcinoma in cirrhosis. PLoS ONE 9(5):e98612
Wildner D, Bernatik T, Greis C, et al. (2015) CEUS in hepatocellular carcinoma and intrahepatic cholangiocellular carcinoma in 320 patients - early or late washout matters: a subanalysis of the DEGUM multicenter trial. Ultraschall Med 36(2):132–139
Ishak K, Baptista A, Bianchi L, et al. (1995) Histological grading and staging of chronic hepatitis. J Hepatol 22:696–699
Park YN (2011) Update on precursor and early lesions of hepatocellular carcinomas. Arch Pathol Lab Med 135:704–715
Coleman WB (2003) Mechanisms of human hepatocarcinogenesis. Curr Mol Med. 3:573–588
International Working Party (1995) Terminology of nodular hepatocellular lesions. Hepatology 22:983–993
Roskams T, Kojiro M (2010) Pathology of early hepatocellular carcinoma: conventional and molecular diagnosis. Semin Liver Dis. 30:17–25
Trevisani F, Cantarini MC, Wands JR, Bernardi M (2008) Recent advances in the natural history of hepatocellular carcinoma. Carcinogenesis 29:1299–1305
Chou CT, Chou JM, Chang TA, et al. (2013) Differentiation between dysplastic nodule and early-stage hepatocellular carcinoma: the utility of conventional MR imaging. World J Gastroenterol 19:7433–7439
Jha RC, Mitchell DG, Weinreb JC, et al. (2014) LI-RADS categorization of benign and likely benign findings in patients at risk of hepatocellular carcinoma: a pictorial atlas. AJR Am J Roentgenol 203:48–69
Choi J-Y, Lee J-M, Sirlin CB (2014) CT and MR imaging diagnosis and staging of hepatocellular carcinoma. Part II. Extracellular agents, hepatobiliary agents, and ancillary imaging features. Radiology 273:30–50
Han J, Liu Y, Han F, et al. (2015) The degree of contrast washout on contrast-enhanced ultrasound in distinguishing intrahepatic cholangiocarcinoma from hepatocellular carcinoma. Ultrasound Med Biol 41(12):3088–3095
Wildner D, Pfeifer L, Goertz RS, et al. (2014) Dynamic contrast-enhanced ultrasound (DCE-US) for the characterization of hepatocellular carcinoma and cholangiocellular carcinoma. Ultraschall Med 35(6):522–527
The Liver Imaging Reporting and Data System (LI-RADS). http://www.acr.org/quality-safety/resources/LIRADS
Kono Y, Cosgrove D, Dietrich C, et al. (2016) Contrast-enhanced ultrasound liver imaging reporting and data system for diagnosis of hepatocellular carcinoma: initial proposal. J Ultrasound Med 35(suppl):S1–S132
Jang HJ, Kono Y, Sirlin C, et al. (2015) Incorporation of CEUS Into LI-RADS for diagnosis of hepatocellular carcinoma (HCC): a work in progress. Radiological Society of North America Annual Meeting. 29 November–4 December 2015, Chicago, IL. GI212-ED-X
Piscaglia F, Wilson SR, Lyshchik A, et al. (2017) American college of radiology contrast enhanced ultrasound liver imaging reporting and data system (CEUS LI-RADS) for the diagnosis of Hepatocellular Carcinoma: a pictorial essay. Ultraschall Med. 38:320–324
Heimbach J, Kulik LM, Finn R, et al. (2017) AASLD guidelines for the treatment of hepatocellular carcinoma. Hepatology. https://doi.org/10.1002/hep.29086
OPTN Policy #9: allocation of livers and liver-intestines. https://optn.transplant.hrsa.gov/media/1200/optn_policies.pdf
Claudon M, Dietrich CF, Choi BI, et al. (2012) A WFUMB-EFSUMB initiative in cooperation with representatives of AFSUMB, AIUM, ASUM, FLAUS and ICUS. Ultrasound Med Biol 2013(39):187–210
Sawhney S, Wilson SR (2017) Can ultrasound with contrast enhancement replace nonenhanced computed tomography scans in patients with contraindication to computed tomography contrast agents? Ultrasound Q 33:125–132
Jang HJ, Kim TK, Burns PN, Wilson SR (2015) CEUS: an essential component in a multimodality approach to small nodules in patients at high-risk for hepatocellular carcinoma. Eur J Radiol 84:1623–1635
Hanna RF, Miloushev VZ, Tang A, et al. (2016) Comparative 13-year meta-analysis of the sensitivity and positive predictive value of ultrasound, CT, and MRI for detecting hepatocellular carcinoma. Abdom Radiol (NY) 41:71–79
Exuzides A, Main ML, Colby C, et al. (2010) A retrospective comparison of mortality in critically ill hospitalized patients undergoing echocardiography with and without an ultrasound contrast agent. JACC Cardiovasc Imaging 3:578–585
Goldberg YH, Ginelli P, Siegel R, et al. (2012) Administration of perflutren contrast agents during transthoracic echocardiography is not associated with a significant increase in acute mortality risk. Cardiology 122(2):119–125
Aggeli C, Giannopoulos G, Roussakis G, et al. (2008) Safety of myocardial flash-contrast echocardiography in combination with dobutamine stress testing for the detection of ischaemia in 5250 studies. Heart 94:1571–1577
Parker JM, Weller MW, Feinstein LM, et al. (2013) Safety of ultrasound contrast agents in patients with known or suspected cardiac shunts. Am J Cardiol 1(112):1039–1045
Jo PC, Jang HJ, Burns PN, et al. (2017) Integration of Contrast-enhanced US into a multimodality approach to imaging of nodules in a cirrhotic liver: how i do it. Radiology 282:317–331
EFSUMB (2010) Minimum training requirements for the practice of medical ultrasound in Europe. Ultraschall in Med 31:426–427
ACR manual on contrast media. https://www.acr.org/~/media/37D84428BF1D4E1B9A3A2918DA9E27A3.pdf
Main ML, Hibberd MG, Ryan A, et al. (2014) Acute mortality in critically ill patients undergoing echocardiography with or without an ultrasound contrast agent. JACC Cardiovasc Imaging 7:40–48
Parker JM, Weller MW, Feinstein LM, et al. (2013) Safety of ultrasound contrast agents in patients with known or suspected cardiac shunts. Am J Cardiol 112:1039–1045
Main ML, Ryan AC, Davis TE, et al. (2008) Acute mortality in hospitalized patients undergoing echocardiography with and without an ultrasound contrast agent (multicenter registry results in 4,300,966 consecutive patients). Am J Cardiol. 102:1742–1746
Wei K, Mulvagh SL, Carson L, et al. (2008) The safety of deFinity and Optison for ultrasound image enhancement: a retrospective analysis of 78,383 administered contrast doses. J Am Soc Echocardiogr 21:1202–1206
Herzog CA (2008) Incidence of adverse events associated with use of perflutren contrast agents for echocardiography. JAMA 299:2023–2025
Muskula PR, Main ML (2017) Safety With echocardiographic contrast agents. Circ Cardiovasc Imaging. https://doi.org/10.1161/CIRCIMAGING.116.005459
Lumason prescribing information. http://imaging.bracco.com/sites/braccoimaging.com/files/technica_sheet_pdf/us-en-2017-01-04-spc-lumason.pdf
Definity prescribing information. http://www.definityimaging.com/pdf/DEFINITY_US_PI_515987-0117.pdf
Yu H, Jang HJ, Kim TK, et al. (2010) Pseudo-enhancement within local ablation zone of hepatic tumors due to nonlinear artifact on contrast-enhanced ultrasound. Am J Roentgenol 194:653–659
Dietrich CF, Ignee A, Greis C, et al. (2014) Artifacts and pitfalls in contrast-enhanced ultrasound of the liver. Ultraschall Med 35(2):108–125
Li SY, Huang P, Cosgrove D, et al. (2016) Pseudoenhancement of gallbladder sludge: a confusing artifact caused by nonlinear propagation of ultrasound through microbubbles. Ultraschall Med 37(3):307–309
Fowler KJ, Potretzke TA, Hope TA, Costa EA, Wilson SR (2017) LI-RADS M (LR-M): definite or probable malignancy, not specific for hepatocellular carcinoma. Abdom Radiol (NY). https://doi.org/10.1007/s00261-017-1196-2
Hanna RF, Aguirre DA, Kased N, et al. (2008) Cirrhosis-associated hepatocellular nodules: correlation of histopathologic and MR imaging features. Radiographics 28:747–769
Acknowledgments
The authors would like to dedicate this manuscript to our dear friend Dr. David Cosgrove (1938–2017). David was a thought leader in the clinical ultrasound and a passionate advocate of CEUS. He was one of the founding members of the CEUS LI-RADS Working Group and spent countless hours working on this project. His extraordinary knowledge, enthusiasm, and dedication were extremely valuable and will be greatly missed. Supported in part by NCI Grant R01 CA215520-01A1.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Conflict of interest
Andrej Lyshchik: Research support: GE, Siemens, Toshiba; Advisory Board: Bracco; Speaker: GE, SonoScape. Yuko Kono: Research support: Toshiba, GE, Lantheus. Christoph F Dietrich: Advisory Board; Hitachi, Mindray, JAZZ. Speaker: Hitachi, Supersonic, Siemens, Mindray, GE, Bracco, Pentax, Novartis. Hyun-Jung Jang: none. Tae Kyoung Kim: none. Fabio Piscaglia: Research support: Esaote; Advisory Board: Bayer, Eisai; Speaker: Bracco, Bayer. Alexander Vezeridis: none. Juergen K Willmann: Research support: Siemens, GE, Philips, Bracco; Consulting: Bracco, Triple Ring Technologies; Advisory Board: Lantheus, Bracco, SonoVol. Stephanie R Wilson: Research support: Siemens, Philips and Samsung; Speaker: GE and Samsung
Rights and permissions
About this article
Cite this article
Lyshchik, A., Kono, Y., Dietrich, C.F. et al. Contrast-enhanced ultrasound of the liver: technical and lexicon recommendations from the ACR CEUS LI-RADS working group. Abdom Radiol 43, 861–879 (2018). https://doi.org/10.1007/s00261-017-1392-0
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00261-017-1392-0