Abstract
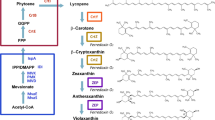
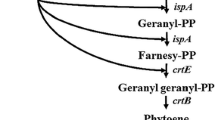
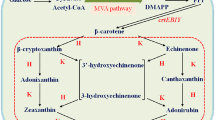
Carotenoids are naturally synthesized in some species of bacteria, archaea, and fungi (including yeasts) as well as all photosynthetic organisms. Escherichia coli has been the most popular bacterial host for the heterologous production of a variety of carotenoids, including even xanthophylls unique to photosynthetic eukaryotes such as lutein, antheraxanthin, and violaxanthin. However, conversion efficiency of these epoxy-xanthophylls (antheraxanthin and violaxanthin) from zeaxanthin remained substantially low. We here examined several factors affecting their productivity in E. coli. Two sorts of plasmids were introduced into the bacterial host, i.e., a plasmid to produce zeaxanthin due to the presence of the Pantoea ananatis crtE, crtB, crtI, crtY, and crtZ genes in addition to the Haematococcus pluvialis IDI gene, and one containing each of zeaxanthin epoxidase (ZEP) genes originated from nine photosynthetic eukaryotes. It was consequently found that paprika (Capsicum annuum) ZEP (CaZEP) showed the highest conversion activity. Next, using the CaZEP gene, we performed optimization experiments in relation to E. coli strains as the production hosts, expression vectors, and ribosome-binding site (RBS) sequences. As a result, the highest productivity of violaxanthin (231 μg/g dry weight) was observed, when the pUC18 vector was used with CaZEP preceded by a RBS sequence of score 5000 in strain JM101(DE3).





Similar content being viewed by others
References
Alper H, Stephanopoulos G (2008) Uncovering the gene knockout landscape for improved lycopene production in E. coli. Appl Microbiol Biotechnol 78:801–810
Araki M, Kaku N, Harada M, Ando Y, Yamaguchi R, Shindo K (2016) Production of auroxanthins from violaxanthin and 9-cis-violaxanthin by acidic treatment, and the antioxidant activities of violaxanthin, 9-cis-violaxanthin, and auroxanthins. J Agric Food Chem 64:9352–9355
Baneyx F (1999) Recombinant protein expression in Escherichia coli. Curr Opin Biotechnol 10:411–421
Bongers M, Chrysanthopoulos PK, Behrendorff JBYH, Hodson MP, Vickers CE, Nielsen LK (2015) Systems analysis of methylerythritol-phosphate pathway flux in E. coli: insights into the role of oxidative stress and the validity of lycopene as an isoprenoid reporter metabolite. Microb Cell Factories 14:193
Borujeni AE, Channarasappa AS, Salis HM (2014) Translation rate is controlled by coupled trade-offs between site accessibility, selective RNA unfolding and sliding at upstream standby sites. Nucleic Acids Res 42:2646–2659
Bouvier F, d’Harlingue A, Hugueney P, Marin E, Marion-Poll A, Camara B (1996) Xanthophyll biosynthesis –cloning, expression, functional reconstitution, and regulation of β-cyclohexenyl carotenoid epoxidase from pepper (Capsicum annuum). J Biol Chem 271:28861–28867
Britton G, Liaaen-Jensen S, Pfander H (2004) Carotenoids handbook. Birkhäuser Verlag, Basel, Boston, Berlin
Chae HS, Kim KH, Kim SC, Lee PC (2010) Strain-dependent carotenoid productions in metabolically engineered Escherichia coli. Appl Biochem Biotechnol 162:2333–2344
Dambek M, Eilers U, Breitenbach J, Steiger S, Büchel C, Sandmann G (2012) Biosynthesis of fucoxanthin and diadinoxanthin and function of initial pathway genes in Phaeodactylum tricornutum. J Exp Bot 63:5607–5612
Deli J, Ösz E (2004) Carotenoid 5,6-, 5,8-, and 3,6-epoxides. Arkivoc is the totally free online journal that reports all aspects of organic chemical research and secondly by organizing the Florida Heterocyclic and Synthetic Conferences (FloHet Conference) which are held at Gainesville, Florida in March (alternate years) and sponsored by IUPAC. (vii):150–168
Eilers U, Dietzel L, Breitenbach J, Büchel C, Sandmann G (2016) Identification of genes coding for functional zeaxanthin epoxidases in the diatom Paeodactylum tricornutum. J Plant Physiol 192:64–70
Harada H, Yu F, Okamoto S, Kuzuyama T, Utsumi R, Misawa N (2009) Efficient synthesis of functional isoprenoids from acetoacetate through metabolic pathway-engineered Escherichia coli. Appl Microbiol Biotechnol 81:915–925
Lee P, Schmidt-Dannert C (2002) Metabolic engineering towards biotechnological production of carotenoids in microorganisms. Appl Microbiol Biotechnol 60:1–11
Li XR, Tian GQ, Shen HJ, Liu JZ (2015) Metabolic engineering of Escherichia coli to produce zeaxanthin. J Ind Mcrobiol Biotechnol 42:627–636
Marz U (2015) “The global market for carotenoids. July 2015”, BCC Research, code FOD025E
Misawa N (2010) Carotenoids. In: Mander L, Lui H-W (eds) Comprehensive natural products II chemistry and biology, vol 1. Elsevier, Oxford, pp 733–753
Misawa N (2011) Pathway engineering for functional isoprenoids. Curr Opin Biotechnol 22:627–633
Misawa N, Satomi Y, Kondo K, Yokoyama A, Kajiwara S, Saito T, Ohtani T, Miki W (1995) Structure and functional analysis of a marine bacterial carotenoid biosynthesis gene cluster and astaxanthin biosynthetic pathway proposed at the gene level. J Bacteriol 177:6575–6584
Misawa N, Shimada S (1998) Metabolic engineering for the production of carotenoids in non-carotenogenic bacteria and yeasts. J Biotechnol 59:169–181
Moise AR, Al-Babili S, Wutzel ET (2014) Mechanistic aspects of carotenoid biosynthesis. Chem Rev 114:164–193
Salis HM (2011) The ribosome binding site calculator. Methods Enzymol 498:19–42
Salis HM, Mirsky EA, Voigt CA (2009) Automated design of synthetic ribosome binding sites to precisely control protein expression. Nat Biotechnol 27:946–950
Shimode S, Miyata K, Araki M, Shindo K (2018) Antioxidant activities of the antheraxanthin-related carotenoids, antheraxanthin, 9-cis-antheraxanthin, and mutatoxanthins. Oleo Sci 67:977–981
Takemura M, Maoka T, Misawa N (2013) Carotenoid analysis of a liverwort Marchantia polymorpha and functional identification of its lycopene β- and ε-cyclase genes. Plant Cell Physiol 55:194–200
Takemura M, Maoka T, Misawa N (2015a) Biosynthetic routes of hydroxylated carotenoids (xanthophylls) in Marchantia polymorpha (liverwort), and production of novel and rare xanthophylls through pathway engineering in Escherichia coli. Planta 241:699–710
Takemura M, Maoka T, Misawa N (2014) Carotenoid analysis of a liverwort Marchantia polymorpha and functional identification of its lycopene β- and ε-cyclase genes. Plant Cell Physiol 55:194–200
Takemura M, Maoka T, Osawa A, Higashinaka H, Shimada H, Shindo K, Misawa N (2015b) (6E) and (6Z)-9’-Aporhodoxanthinone, novel carotenoids produced in zeaxanthin-synthesizing-Escherichia coli by redox stress. Tetrahedron Lett 56:6063–6065
Takemura M, Tanaka R, Misawa N (2017) Pathway engineering for the production of β-amyrin and cycloartenol in Escherichia coli -a method to biosynthesize plant-derived triterpene skeletons in E. coli. Appl Microbiol Biotechnol 101:6615–6625
Tashiro M, Fujii A, Kawai-Noma S, Saito K, Umeno D (2017) Directed evolution and expression tuning of geraniol synthase for efficient geraniol production in Escherichia coli. J Gen Appl Microbiol 63:287–295
Yokoyama A, Miki W (1995) Composition and presumed biosynthetic pathway of carotenoids in the astaxanthin-producing bacterium Agrobacterium aurantiacum. FEMS Microbiol Lett 128:139–144
Yoon SH, Kim JE, Lee SH, Park HM, Choi MS, Kim JY, Lee SH, Shin YC, Keasling JD, Kim SW (2007) Engineering the lycopene synthetic pathway in E. coli by comparison of the carotenoid genes of Pantoea agglomerans and Pantoea ananatis. Appl Microbiol Biotechnol 74:131–139
Yoon S, Lee S, Amitabha D, Ryu H, Jang H, Kim J, Oh D, Keasling JD, Kim S (2009) Combinatorial expression of bacterial whole mevalonate pathway for the production of β-carotene in E. coli. J Biotechnol 140:218–226
Zhu C, Yamamura S, Nishihara M, Koiwa H, Sandmann G (2003) cDNAs for the synthesis of cyclic carotenoids in petals of Gentiana lutea and their regulation during flower development. Biochim Biophys Acta 1625:305–308
Acknowledgments
We thank Dr. Changfu Zhu and Dr. Gerhard Sandmann for giving us the GlZEP cDNA clone. We also thank Dr. Hisashi Harada for giving us the Paeodactylum tricornutum genome DNA. The authentic samples violaxanthin and antheraxanthin are gifts from Dr. Kazutoshi Shindo.
Funding
This study was funded by the “Smart Cell Project” organized by the New Energy and Industrial Technology Development Organization (NEDO) (16100920-0).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
This article does not contain any studies with animals performed by any of the authors.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Takemura, M., Kubo, A., Higuchi, Y. et al. Pathway engineering for efficient biosynthesis of violaxanthin in Escherichia coli. Appl Microbiol Biotechnol 103, 9393–9399 (2019). https://doi.org/10.1007/s00253-019-10182-w
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-019-10182-w